Researchers Find Way to Tune Thermal Conductivity of 2-D Materials
For Immediate Release
Researchers have found an unexpected way to control the thermal conductivity of two-dimensional (2-D) materials, which will allow electronics designers to dissipate heat in electronic devices that use these materials.
2-D materials have a layered structure, with each layer having strong bonds horizontally, or “in plane,” and weak bonds between the layers, or “out of plane.” These materials have unique electronic and chemical properties, and hold promise for use in creating flexible, thin, lightweight electronic devices.
For many of these potential applications, it’s important to be able to dissipate heat efficiently. And this can be tricky. In 2-D materials, heat is conducted differently in plane than it is out of plane.
For example, in one class of 2-D materials, called TMDs, heat is conducted at 100 watts per meter per Kelvin (W/mK) in plane, but at only 2 W/mK out of plane. That gives it a “thermal anisotropy ratio” of about 50.
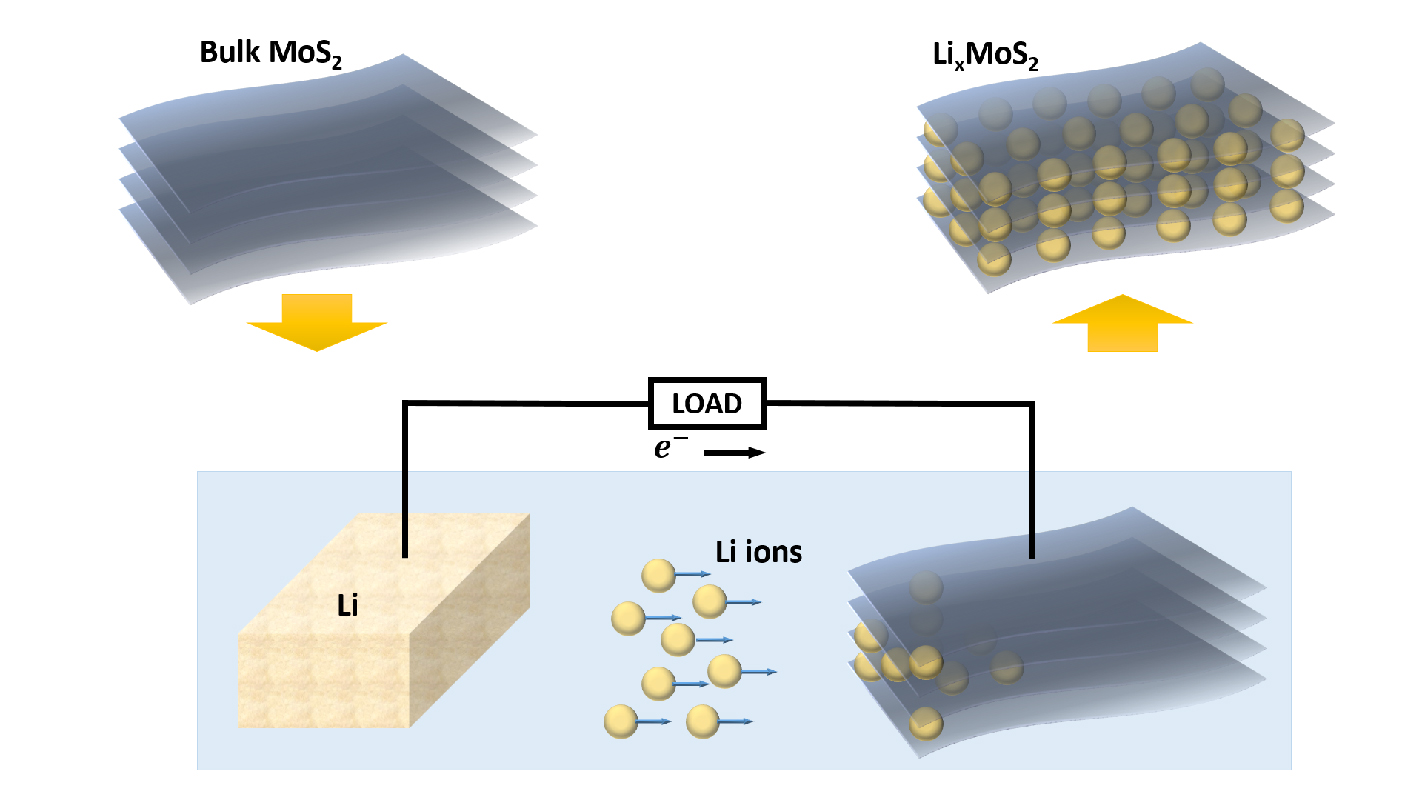
To better understand the thermal conduction properties of 2-D materials, a team of researchers from North Carolina State University, the University of Illinois at Urbana-Champaign (UI) and the Toyota Research Institute of North America (TRINA) began experimenting with molybdenum disulfide (MoS2), which is a TMD.
The researchers found that, by introducing disorder to the MoS2, they could significantly alter the thermal anisotropy ratio.
The researchers created this disorder by introducing lithium ions between the layers of MoS2. The presence of the lithium ions does two things simultaneously: it puts the layers of the 2-D material out of alignment with each other, and it forces the MoS2 to rearrange the structure of its component atoms.
When the ratio of lithium ions to MoS2 reached 0.34, the in-plane thermal conductivity was 45 W/mK, and the out-of-plane thermal conductivity dropped to 0.4 W/mK– increasing the material’s thermal anisotropy ratio from 50 to more than 100. In other words, heat became more than twice as likely to travel in plane – along the layer, rather than between the layers.
And that was as good as it got. Adding fewer lithium ions made the thermal anisotropy ratio lower. Adding more ions also made it lower. But in both cases, the ratio was affected in a predictable way, meaning that the researchers could tune the material’s thermal conductivity and thermal anisotropy ratio.
“This finding was very counter-intuitive,” says Jun Liu, an assistant professor of mechanical and aerospace engineering at NC State and co-corresponding author of a paper describing the work. “The conventional wisdom has been that introducing disorder to any material would decrease the thermal anisotropy ratio.
“But based on our observations, we feel that this approach to controlling thermal conductivity would apply not only to other TMDs, but to 2-D materials more broadly,” Liu says.
“We set out to advance our fundamental understanding of 2-D materials, and we have,” Liu adds. “But we also learned something that is likely to be of practical use for the development of technologies that make use of 2-D materials.”
The paper, “Tuning Thermal Conductivity in Molybdenum Disulfide by Electrochemical Intercalation,” is published in the journal Nature Communications. Co-corresponding authors of the paper are Gaohua Zhu of TRINA and David Cahill of UI. Co-authors are Ruigang Zhang and Debasish Banerjee of TRINA, and Qiye Zheng and Dongyao Li of UI. The work was supported by TRINA.
-shipman-
Note to Editors: The study abstract follows.
“Tuning Thermal Conductivity in Molybdenum Disulfide by Electrochemical Intercalation”
Authors: Gaohua Zhu, Ruigang Zhang, and Debasish Banerjee, Toyota Research Institute of North America; Jun Liu, North Carolina State University; Qiye Zheng, Dongyao Li, and David G. Cahill, University of Illinois at Urbana-Champaign
Published: Oct. 21, Nature Communications
DOI: 10.1038/NCOMMS13211
Abstract: The thermal conductivity of lithium ion intercalated molybdenum disulfide (LixMoS2) is measured as a function of the degree of lithiation (x), using time-domain thermoreflectance. We show that the thermal conductivity can be modified by electrochemical intercalation. We investigated two types of LixMoS2 samples with different orientations of the basal planes: i) thin films with vertically-aligned basal planes created by sulfurization of molybdenum (Mo); and ii) natural bulk crystals with basal planes parallel to the surface. The thermal conductivity of thin film MoS2 can be tuned from ≈3.4 to ≈1.7 W m-1 K-1 with lithium ion intercalation. The thermal conductivity of bulk MoS2 can be modulated from ≈2.0 to ≈0.4 W m-1 K-1 and ≈105 to ≈45 W m-1 K-1, along the through-plane and in-plane directions, respectively. We characterized the Raman-active vibrational modes, lattice expansion, and elastic constants. The change of thermal conductivity correlates with the structural and compositional disorder, e.g., the stacking order of the layered structure and the lithiation-dependent semiconductor (2H) to metal (1T) phase transition. We found that the ratio of the in-plane to through-plane thermal conductivity is enhanced by the disorder in bulk crystals. These results suggest that stacking disorder and mixture of phases is an effective mechanism to modify the anisotropic thermal conductivity of 2D materials.
- Categories:


