Green Approach Accelerates Process Optimization and Retrieval of ‘Switchable’ Solvents
For Immediate Release
Researchers at North Carolina State University have demonstrated a new, green technology for both accelerated screening and retrieving “switchable” solvents used in green chemistry applications. The new approach makes the screening process hundreds of times faster and drastically accelerates the rate at which solvents can be retrieved from solution.
“We have effectively created a platform that makes green chemistry greener,” says Milad Abolhasani, an assistant professor of chemical and biomolecular engineering at NC State and corresponding author of a paper on the work. “This work expedites industry’s ability to identify the best switchable solvent for a specific chemical process and then gives industry advanced tools to retrieve that solvent far more quickly than is possible using previous approaches.”
At issue are switchable solvents, which change their physicochemical properties when exposed to carbon dioxide (CO2). This study focused on solvents that become hydrophilic in the presence of CO2 and water, but are hydrophobic when CO2 is removed. This makes them attractive for use in chemical and pharmaceutical industry processes, because the solvent can be easily removed from the product by adding CO2 and water. The solvent can then be reclaimed from the water by removing the CO2.
“However, from an industrial point of view, there are significant challenges,” Abolhasani says. “Specifically, the process for screening candidates to identify the most efficient switchable solvent for a particular application can be extremely time- and labor-intensive. And once you have the right switchable solvent candidate, removing it on a large scale can also take a long time.”
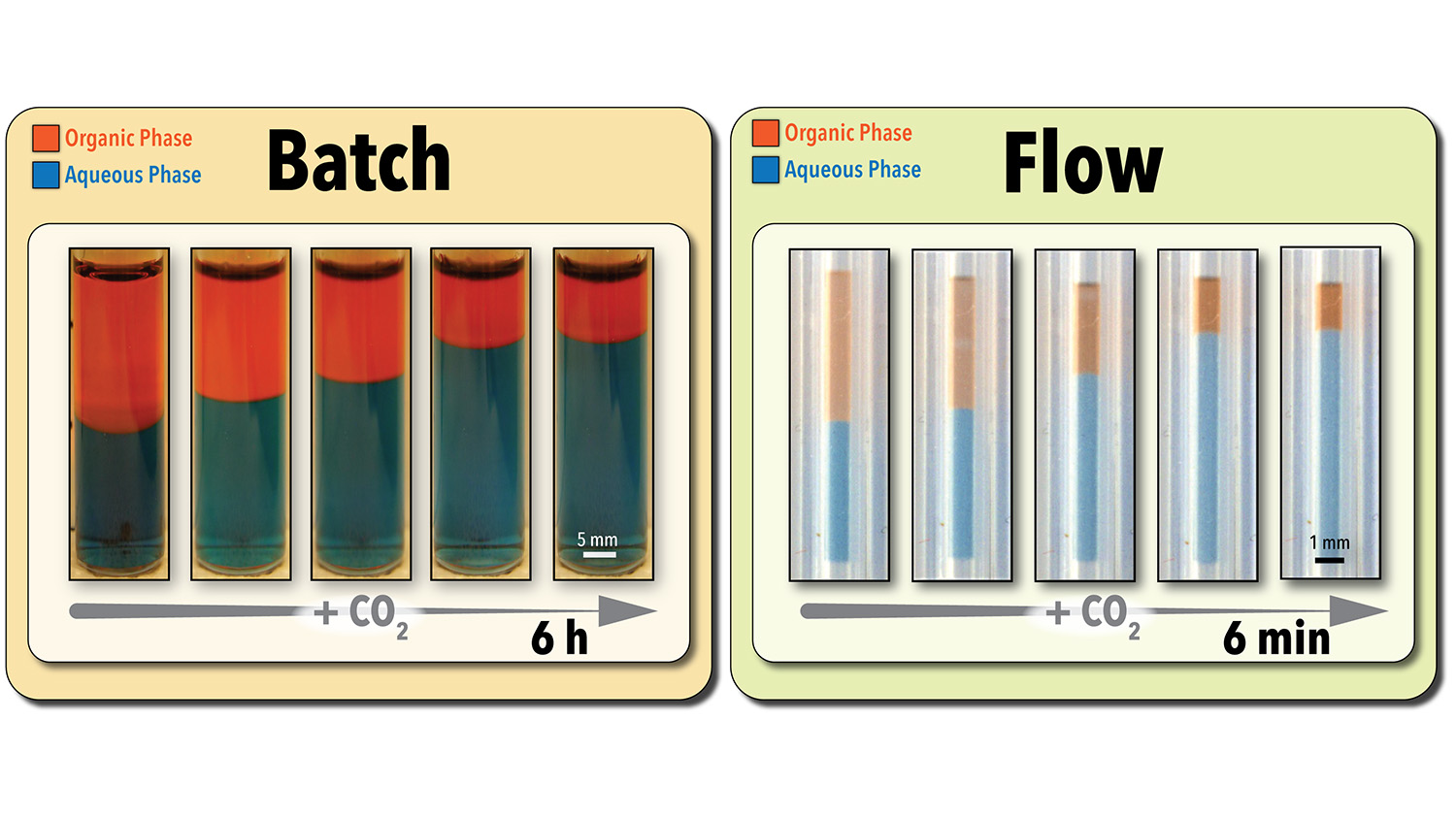
To address the screening problem, Abolhasani’s team made use of a microscale flow chemistry platform that runs 5-microliter samples through a gas-permeable tube that is surrounded by CO2. This ensures the solvent in the sample is in constant contact with the CO2 (i.e., intensified mass transfer), accelerating the reaction and the solvent recovery process.
Using this technique, the researchers can determine a solvent’s efficiency, using image processing, in as little as three minutes. The platform also allows them to run multiple samples simultaneously. Accounting for the time needed to prepare each sample, the system allows users to run approximately 280 screening experiments per day.
By comparison, conventional batch testing techniques require the use of larger sample sizes. For example, testing the efficiency of a 5-milliliter sample using conventional batch techniques takes between six and eight hours – or approximately one test per day.
Abolhasani’s team also demonstrated in experimental testing that the flow chemistry technique was not only faster, but was just as accurate as conventional batch testing at determining a solvent’s efficiency.
In addition, the researchers have recently shown that they can reconfigure the same flow chemistry platform utilized for rapid switchable solvent screening into a continuous flow mode for retrieving solvents on a large scale. “Our approach is also more cost effective in that it is completely computer-controlled and is, therefore, less labor-intensive,” Abolhasani says.
“We’re excited about the potential of this process intensification technology and are looking for partners to help us transfer the technique from the lab to industrial R&D and manufacturing applications.”
The paper, “Accelerated Material-Efficient Investigation of Switchable Hydrophilicity Solvents for Energy-Efficient Solvent Recovery,” is published in the journal ACS Sustainable Chemistry & Engineering. First author of the paper is Suyong Han, a Ph.D. student at NC State. The paper was co-authored by Keshav Raghuvanshi, a postdoctoral researcher at NC State. The work was done with support from the American Chemical Society Petroleum Research Fund, under grant number 59602-DNI9.
-shipman-
Note to Editors: The study abstract follows.
“Accelerated Material-Efficient Investigation of Switchable Hydrophilicity Solvents for Energy-Efficient Solvent Recovery”
Authors: Suyong Han, Keshav Raghuvanshi and Milad Abolhasani, North Carolina State University
Published: Feb. 6, ACS Sustainable Chemistry & Engineering
DOI: 10.1021/acssuschemeng.9b07304
Abstract: In most chemical industries, solvent removal and recovery processes are heavily dependent on hazardous volatile solvents with energy-intensive distillation processes due to their ease of separation. An emerging promising alternative is implementing switchable solvents with on-demand and reversible switching of their physiochemical properties triggered by carbon dioxide (CO2). Utilization and widespread implementation of switchable solvents can dramatically reduce environmental risks and energy requirements for solvent removal and recovery processes. Despite intriguing characteristics of switchable solvents, time- and material-intensive nature of conventional batch strategies have hindered a comprehensive understanding of this exciting class of green solvents. Herein, we report an accelerated time- and material-efficient (green) flow chemistry strategy for in-situ fundamental and applied studies of CO2-mediated switchable solvents. Utilizing a highly gas-permeable membrane microreactor increases the gas-liquid interfacial area for CO2 injection, thereby enhancing the gas-liquid mass transfer (~60 times faster equilibrium time than a batch reactor), while minimizing the chemical consumption (~1000 times less than a batch reactor) and waste generation (~1500 times less than a batch reactor) for each solvent switching experiment. Utilizing the developed green flow chemistry strategy, we comprehensively study the effects of continuous and discrete process parameters on the efficiency and kinetics of hydrophilicity switching of switchable solvents. The intensified CO2-triggered SHS extraction process allows accurate material-efficient studies of switchable solvents, and therefore will accelerate the development and adoption of distillation-free, green, and sustainable solvent recovery strategies in chemical industries.


