For Immediate Release
In a small pilot study, researchers from North Carolina State University have demonstrated a rapid, simple way to generate large numbers of lung stem cells for use in disease treatment. This method of harvesting and growing a patient’s own lung stem cells shows promise in mice for treating idiopathic pulmonary fibrosis (IPF), and could one day provide human IPF sufferers with an effective, less invasive method of treatment for their disease.
The idea of using the body’s own cells to fight diseases is not new, but current methods of isolating stem cells from bone marrow, fat tissue or cord blood are time consuming, costly and often wasteful. “In current stem cell harvesting, just the process of sorting the stem cells can damage them, wasting not only the cells, but also time and money,” says Ke Cheng, lead researcher on the project. “We wanted to see if we could take healthy stem cells from an organ while they were still in a supportive environment, recreate and enhance that environment outside the body to encourage stem cell reproduction, then reintroduce those cells into a damaged organ to treat disease.” Cheng is an associate professor of regenerative medicine at NC State’s College of Veterinary Medicine and the UNC-Chapel Hill/NC State joint department of biomedical engineering.
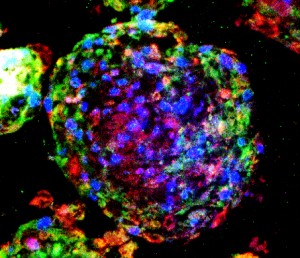
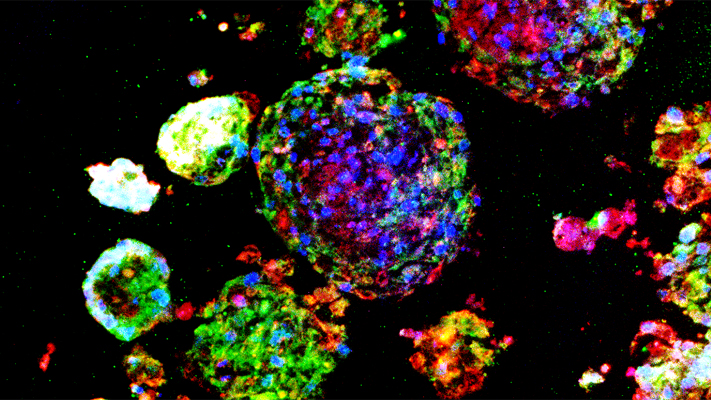
Instead of attempting to isolate and sort individual lung stem cells or genetically convert other types of cells into lung stem cells, Cheng used a multicellular spheroid method to harvest and grow them. A spheroid is a three-dimensional cellular structure that has typically been used to culture cancer cells or embryonic stem cells for experimentation and research. The cells of interest – in Cheng’s research, lung stem cells – are at the center of the spheroid, and they are surrounded by layers of supporting cells.
“We’re the first lab to show that the spheroid environment can be used to enrich adult lung stem cells. In the spheroid, we recreate the stem cells’ natural microenvironment, the “niche,” where they can communicate with each other just as they would inside your body,” says Cheng. “There is no use of exogenous or transgenic materials – the stem cells are 100 percent the donor’s own genetic material, a perfect match to the patient and the organ being treated.”
In a small animal trial, Cheng and his graduate students tested the spheroid-produced human lung stem cells on mice with IPF, a fatal disease that thickens and scars healthy lung tissue, creating inflammation and replacing the lining of the lung cells with fibroids. “This was a proof of concept trial. The mice that received the stem cell transplant showed decreases in inflammation and fibrosis – their lungs almost matched those of the control group, who did not have IPF at all,” says Cheng. “And the beauty of the process is that the cell therapy can be delivered intravenously.
“Picture the lung as a garden and the stem cells as seeds. In an IPF environment, with inflammation, the soil is bad, but the seeds are still there,” adds Cheng. “We take the seeds out and give them a protected place to grow. Then when we put them back into the lung, they can grow into mature lung cells to replace the damaged lung tissues in IPF. They can also wake the other seeds up, telling them to help fight the inflammation and ‘improving’ the soil.”
Cheng’s next steps will be to see if potent stem cells can be harvested and grown from biopsied tissues of IPF patients, which would further reduce the number of invasive procedures a patient would need to endure. “We’ve demonstrated that the spheroid is a wonderful method for stem cell production – now we want to try and harvest the original cells during the biopsy,” he says. “Of course, our ultimate goal is to use this method to treat humans with IPF.”
The research appears in STEM CELLS Translational Medicine. The work was supported by the American Heart Association (grant 12BGIA12040477), NC State University’s Chancellor’s Faculty Excellence Program, the NC State Center for Comparative Medicine and Translational Research and the Kenan Foundation Regenerative Medicine Grant. NC State graduate students Eric Henry, Jhon Cores, Taylor Hensley, Shirena Anthony, Tyler Allen, James de Andrade and Adam Vandergriff, as well as Drs. Thomas Caranasos and Jason Lobo from the University of North Carolina at Chapel Hill contributed to the work.
-peake-
Note to editors: Abstract follows.
“Adult Lung Spheroid Cells Contain Progenitor Cells and Mediate Regeneration in Rodents with Bleomycin-Induced Pulmonary Fibrosis”
Authors: Eric Henry, Jhon Cores, M. Taylor Hensley, Shirena Anthony, Adam Vandergriff, Tyler Allen, James B.M. de Andrade, Ke Cheng, NC State University; Thomas G. Caranasos, L. Jason Lobo, University of North Carolina Chapel Hill
Published: Online in STEM CELLS Translational Medicine
Abstract:
Lung diseases are devastating conditions and ranked as one of the top five causes of mortality worldwide according to the World Health Organization. Stem cell therapy is a promising strategy for lung regeneration. Previous animal and clinical studies have been focused on the use of mesenchymal stem cells (from other parts of the body) for lung regenerative therapies. Here we report a rapid and robust method to generate therapeutic resident lung progenitors from adult lung tissues. Outgrowth cells from healthy lung tissue explants are self-aggregated into three-dimensional lung spheroids in a suspension culture. Without antigenic sorting, lung spheroids recapitulate stem cell niche and contain a natural mixture of lung stem cells and supporting cells. In vitro, lung spheroid cells can be expanded to a large quantity and can form alveoli-like structures and acquire mature lung epithelial phenotypes. In severe combined immunodeficiency mice with bleomycin-induced pulmonary fibrosis, intravenous injection of human lung spheroid cells inhibits apoptosis, fibrosis, and infiltration, but promote angiogenesis. In a syngeneic rat model of pulmonary fibrosis, lung spheroid cells outperforms adipose-derived mesenchymal stem cells in reducing fibrotic thickening and infiltration. Previously the spheroid model has only been used to study lung cancer cells. Our data suggest lung spheroids and lung spheroid cells from healthy lung tissues as great sources of regenerative lung cells for therapeutic lung regeneration.
- Categories: