Mucosal Environment of Older Pigs Helps Newborn Piglets With Intestinal Injury Recover
For Immediate Release
Researchers from North Carolina State University have found that the intestinal mucosal environment of juvenile pigs can stimulate repair of intestinal injuries in newborn piglets. The findings have implications both for understanding why newborns of many species – including humans – are unable to repair these injuries on their own, as well as for potential future treatments.
Intestinal ischemic injury occurs when blood flow to a portion of the intestine is cut off, resulting in the loss of epithelial cells that line intestinal walls. Once this barrier is damaged, intestinal contents can leak into the bloodstream, causing sepsis and often fatal infections. Infants are particularly vulnerable to these injuries; this research shows it may be because they lack the ability to quickly repair the damaged areas.
“In these intestinal injuries, the epithelium sloughs off and creates small holes through which bacteria enter the bloodstream,” says Amanda Ziegler, NC State postdoctoral researcher and lead author of a paper describing the research. “Older animals and human adults can repair these holes within minutes to hours, but newborn pigs cannot. We want to understand the repair mechanism – or lack of it – in newborns.”
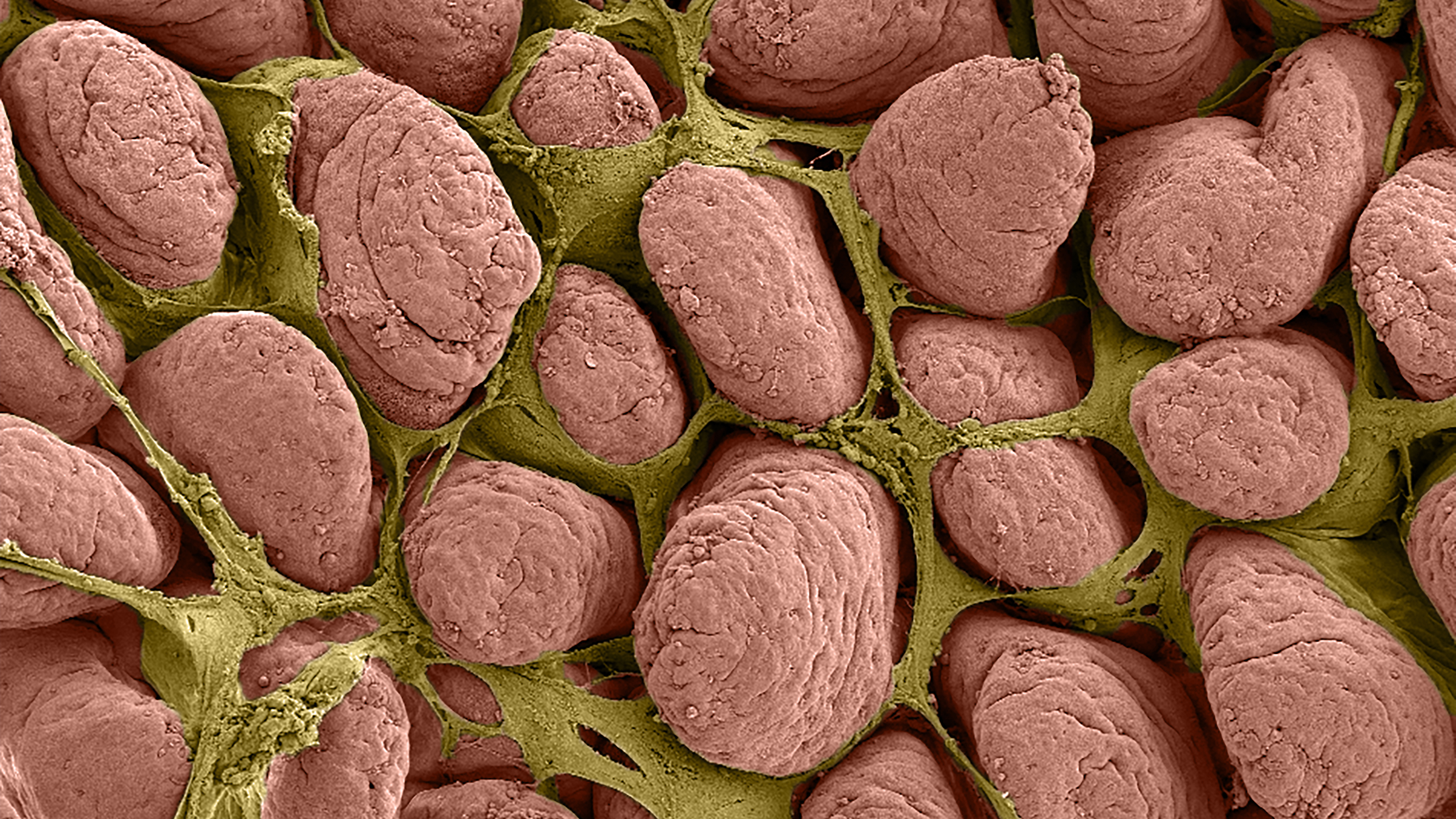
Ziegler and her team looked at ischemia in 2-week-old and 6-week-old pigs by surgically inducing ischemia in small sections of intestine and then removing the injured tissue for study. They noted that in the juvenile (or 6-week-old) pigs, the uninjured epithelial cells flattened out and resealed the intestinal barrier within a couple of hours. The epithelial cells from the newborn pigs, however, did not flatten or reseal the barrier.
The researchers then scraped the mucosal micro-environment – essentially the surface layer – from the 6-week-old intestine and applied it to the newborn intestine, which was then able to repair itself. “There’s a signal here that the newborn is either not getting or not able to respond to,” Ziegler says. “Something, or possibly a number of things within this environment, are working to help this process along.”
The researchers’ next steps will be to look closely at how the epithelial cells “learn” to repair these injuries.
“This work is a first step toward understanding what is happening on the cellular level in young patients with ischemic intestinal injuries,” Ziegler says. “Plus we’ve shown that we can rescue this tissue, although we don’t yet understand the mechanisms behind it. Hopefully future studies will reveal those mechanisms, and lead to better treatments to help improve patient survival rates.”
The research appears in PLOS ONE and was funded by the National Institutes of Health (grant T32 5T32DK007737-22) and the U.S. Department of Agriculture National Institute of Food and Agriculture (award 2017-67015-26804). Anthony Blikslager, professor of equine surgery and gastroenterology at NC State, is corresponding author. Other NC State contributors are: Laurianne Van Landeghem, assistant professor of molecular biomedical sciences; Jack Odle, William Neal Reynolds Professor of Nutritional Biochemistry; veterinary student Juliana Mills; Liara Gonzalez, assistant professor of gastroenterology and equine surgery; and Blikslager lab manager Tiffany Pridgen.
-peake-
Note to editors: An abstract of the paper follows.
“Epithelial restitution defect in neonatal jejunum is rescued by juvenile mucosal homogenate in a pig model of intestinal ischemic injury and repair”
DOI: 10.1371/journal.pone.0200674
Authors: Amanda L. Ziegler, Tiffany A. Pridgen, Juliana K. Mills, Liara M. Gonzalez, Laurianne Van Landeghem, Jack Odle, Anthony T. Blikslager, North Carolina State University
Published: PLOS ONE
Abstract:
Intestinal ischemic injury results sloughing of the mucosal epithelium leading to host sepsis and death unless the mucosal barrier is rapidly restored. Volvulus and neonatal necrotizing enterocolitis (NEC) in infants have been associated with intestinal ischemia, sepsis and high mortality rates. We have characterized intestinal ischemia/ repair using a highly translatable porcine model in which juvenile (6-8-week-old) pigs completely and efficiently restore barrier function by way of rapid epithelial restitution and tight junction re-assembly. In contrast, separate studies showed that younger neonatal (2-week-old) pigs exhibited less robust recovery of barrier function, which may model an important cause of high mortality rates in human infants with ischemic intestinal disease. Therefore, we aimed to further refine our repair model and characterize defects in neonatal barrier repair. Here we examine the defect in neonatal mucosal repair that we hypothesize is associated with hypomaturity of the epithelial and subepithelial compartments. Following jejunal ischemia in neonatal and juvenile pigs, injured mucosa was stripped from seromuscular layers and recovered ex vivo while monitoring transepithelial electrical resistance (TEER) and 3H-mannitol flux as measures of barrier function. While ischemia-injured juvenile mucosa restored TEER above control levels, reduced flux over the recovery period and showed 93±4.7% wound closure, neonates exhibited no change in TEER, increased flux, and a 11±23.3% increase in epithelial wound size. Scanning electron microscopy revealed enterocytes at the wound margins of neonates failed to assume the restituting phenotype seen in restituting enterocytes of juveniles. To attempt rescue of injured neonatal mucosa, neonatal experiments were repeated with the addition of exogenous prostaglandins during ex vivo recovery, ex vivo recovery with full thickness intestine, in vivo recovery and direct application of injured mucosal homogenate from neonates or juveniles. Neither exogenous prostaglandins, intact seromuscular intestinal layers, nor in vivo recovery enhanced TEER or restitution in ischemia-injured neonatal mucosa. However, ex vivo exogenous application of injured juvenile mucosal homogenate produced a significant increase in TEER and enhanced histological restitution to 80±4.4% epithelial coverage in injured neonatal mucosa. Thus, neonatal mucosal repair can be rescued through direct contact with the cellular and non-cellular milieu of ischemia-injured mucosa from juvenile pigs. These findings support the hypothesis that a defect in mucosal repair in neonates is due to immature repair mechanisms within the mucosal compartment. Future studies to identify and rescue specific defects in neonatal intestinal repair mechanisms will drive development of novel clinical interventions to reduce mortality in infants affected by intestinal ischemic injury.
- Categories:


