A Revolution in Regenerative Medicine — Led by NC State
Researchers from across disciplines are developing new approaches and new materials for creating soft tissues.

Every year in the United States, hundreds of thousands of people learn that the pain in their knee or shoulder is a soft tissue that needs repair or replacement.
Surgical advances have made the process of replacing those injured tendons and ligaments fairly routine. But the science behind creating new soft tissues (including skin) has moved forward more slowly.
And that’s where an interdisciplinary team of researchers at NC State University and the University of North Carolina at Chapel Hill has found a potentially transformative opportunity. Drawn from biomedical and industrial engineering, textiles and veterinary medicine, the group is exploring how to apply 3D printing and nonwoven fiber manufacturing to create new tissues that can grow in the human body.
There are three components in tissue engineering, according to the National Institute of Biomedical Imaging and Bioengineering (NIBIB): scaffolds, cells and active molecules that encourage further cell growth. The three work together to generate new tissues, with the scaffold giving form and structure to the tissue growth catalyzed by the active molecules.
Two closely related questions have led the team’s work: How can you manufacture tissues at several scales, from micro to nano, with speed and repeatability? And what should those scaffolds be made of?

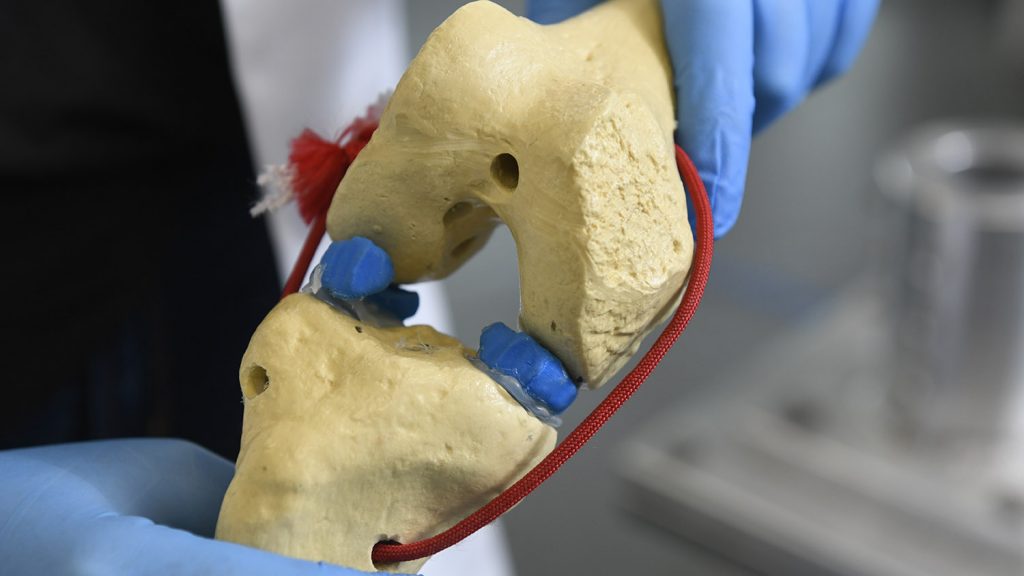
The focus has been on creating scaffolds, which give form and direction to tissue growth. Different tissues have different shapes and different compositions at multiple scales.
“In the absence of scaffolds,” said Rohan Shirwaiker, an associate professor of industrial and systems engineering, “we could still get bone cells and grow them on a petri dish. And they will multiply, but they won’t really grow and form the bone tissue that we need.”
Today, most transplanted tissues come from cadavers or the patients themselves. There’s been some progress on using stem cells to repair damaged tissues. Neither approach delivers the level of customization that the human body demands, said Fran Ligler, Lampe Distinguished Professor in the joint Department of Biomedical Engineering at NC State and UNC-Chapel Hill.
That’s where the Forging Interdisciplinary Bio-inspired Engineered Regenerative Science (FIBERS) team saw its opportunity.
“Once we get a handle on both the materials and the manufacturing, we can really leapfrog what’s going on in the regenerative medicine community,” Ligler said.
3D Printing Meets Nonwoven Materials
To meet the challenge facing tissue engineering, the FIBERS team is using two technologies where NC State has particular strength: 3D printing and nonwoven textiles.
With a 3D printer, a researcher can precisely reproduce the shapes and structures in an MRI image or a CT scan. That control is essential, Shirwaiker said, but traditional 3D printers may not appropriately capture features at the tiny scale that tissue engineering demands.

“With conventional 3D printing, that’s where you run into roadblocks,” Shirwaiker said. “The feature sizes that you can make can be an order of magnitude too large.”
That’s a particular problem at points where engineered tissues meet existing ones. Shirwaiker and Matt Fisher, an assistant professor of biomedical engineering, have been exploring different 3D printing strategies to make tissues such as the meniscus and tendons. At the point where tendon meets bone, Fisher said, there’s an abrupt transition from the stringy, fibrous texture of the soft tissue to the bone.
“That’s what makes it a very difficult engineering problem,” he said.




Eventually, the team reached the limits of what existing 3D printing and nonwoven manufacturing could do. So they built their own machine, with support from the Game-Changing Research Incentive Program (GRIP), a partnership of the NC State Office of Research and Innovation, RTI International, and the Kenan Institute for Engineering, Technology and Science.
What they built is essentially an advanced 3D printer. The chief difference between it and traditional 3D printers is the way it forms fibers, said Benham Pourdeyhimi, executive director of the Nonwovens Institute and the principal investigator for the FIBERS project.
The FIBERS team designed its machine to offer more variety in the size, shape and orientation of the layers of fibers that ultimately form an object. That increased control over architecture, orientation and size allows the team to build structures that better match the natural fibers they’re aiming to replace and regrow. It’s also the most significant advance to come out of the project so far, Pourdeyhimi said, and there’s a patent pending on the features of the process. The team has also applied for a second patent on the specific fiber geometry they’ve been able to produce with the machine.
“What we learned on the little GRIP machine we could never do on a big pilot machine easily,” Pourdeyhimi said. “So for me there were a couple of ‘Aha!’ moments. ‘Wow, if I could do that on a larger scale, it opens up opportunities outside of this domain for other applications.’”
While working through those issues of how to engineer new scaffolds, the team also wrestled with what they should be made of. A scaffold’s mission is fleeting and sensitive. Once implanted, it needs to carry load, spark and shape cell growth, recruit other cells from inside the body, then disappear when the new tissue is able to function alone. And it needs to do all that without disrupting any of the cells and systems around it.
Numerous factors determine whether a given material is up to that job, said Behnam Pourdeyhimi:
- Solidity: Are the pores in the material large enough to allow tissue growth?
- Processing temperature: Can the scaffold material be manufactured at a low enough temperature to avoid damaging cells mixed with it?
- And, if we have to resort to solution blowing, how does it interact with solvents?
To develop a material that meets those needs, Pourdeyhimi needed to reverse a lot of what informs the most common projects at the Nonwovens Institute. Commercial air and water filters, which make up a strategic share of NWI work, demand large amounts of materials with tiny pores.
“We’re learning how to process materials that we’ve never processed before,” Pourdeyhimi said. “We’ve learned how to manipulate them and use more biofriendly types of polymers that the industry would need to use.”
An Interdisciplinary Approach
Tissue engineering itself sits at the intersection of several disciplines: biology, textiles and medicine, to name three. To tackle the field’s grand challenges, the team needed to cross academic boundaries itself.
“We work in a collaborative manner because no single individual can solve the problems that we’re solving,” Pourdeyhimi said.
Fisher is part of the faculty cluster in translational regenerative medicine, and his work with Shirwaiker on 3D printing tissues predates the FIBERS initiative. Pourdeyhimi and Ligler invited them to form the core team.



As the team coalesced, the researchers found that they didn’t just bring different skills. They spoke different languages, Fisher said. The word “scale” is a good example. In his own lab, Fisher spends hours creating a single scaffold with a surface area of a few millimeters at most. His challenge was scaling that painstaking process up “so we can produce a whole bunch of scaffolds at a relatively low cost,” he said.
“We talked to Dr. Pourdeyhimi about scaling up,” Fisher added. “And he said, ’No, what you’re talking about is scaling down.’ When they talk about scale, he’s used to producing large volumes of materials in a single day.”
The team, which also includes faculty from the College of Veterinary Medicine at NC State and the UNC School of Pharmacy, the UNC Burn Center, and orthopaedics department at UNC, meets weekly. The tone of those meetings is different from other academic endeavors Ligler has experienced.
“There’s a real give-and-take between the people on the manufacturing side, in nonwovens, and in biology,” she said. “‘What do you need? What are the metrics? Can you test these for us?’ It’s very iterative.”
The Future of Fibers
To this point, the research team’s work has focused on innovations that would improve quality of life for the hundreds of thousands of people who get replacement soft tissues each year. But they know there’s another population with a greater need.
Right now, roughly 114,000 Americans are waiting for organ transplants, according to the Organ Procurement and Transplantation Network. Based on averages from the last decade, less than a third of them will find a donor this year.
Their options are limited, and their odds are long. It’s for them that engineered tissues and organs hold the greatest promise. The NC State and UNC-CH research team is seeking support to meet their needs.
The FIBERS investigators have requested funding from the National Science Foundation to establish a national hub for regenerative tissue engineering at NC State. That hub would bring together nine other universities and industry partners to build major facilities based on the GRIP concept and create a central library and database of new materials and specifications for scaffolds, with which the research community can interact and collaborate on the development of new structures.
It would also develop the capability to produce, on a mass scale, scaffolds that could grow a range of different tissue types, from anterior cruciate ligaments for young athletes whose only options are tissues from cadavers, to regenerated livers for transplant-seeking patients who may have no other options at all.
“I think this is going to snowball,” Pourdeyhimi said. “I hope it’s going to snowball into something much bigger for our team, for NC State, and for the nation.”



