Researchers Find Simpler Way to Deposit Magnetic Iron Oxide onto Gold Nanorods
For Immediate Release
Researchers from North Carolina State University and MIT have found a simpler way to deposit magnetic iron oxide (magnetite) nanoparticles onto silica-coated gold nanorods, creating multifunctional nanoparticles with useful magnetic and optical properties.
Gold nanorods have widespread potential applications because they have a surface plasmon resonance – meaning they can absorb and scatter light. And by controlling the dimensions of the nanorods, specifically their aspect ratio (or length divided by diameter), the wavelength of the absorbed light can be controlled. This characteristic makes gold nanorods attractive for use in catalysis, security materials and a host of biomedical applications, such as diagnostics, imaging, and cancer therapy. The fact that the magnetite-gold nanoparticles can also be manipulated using a magnetic field enhances their potential usefulness for biomedical applications, such as diagnostic tools or photothermal therapeutics.
“The approach we outline in our new paper is simple, likely making it faster and less expensive than current techniques for creating these nanoparticles – on a small scale or a large one,” says Joe Tracy, an associate professor of materials science and engineering at NC State and corresponding author of a paper on the work.
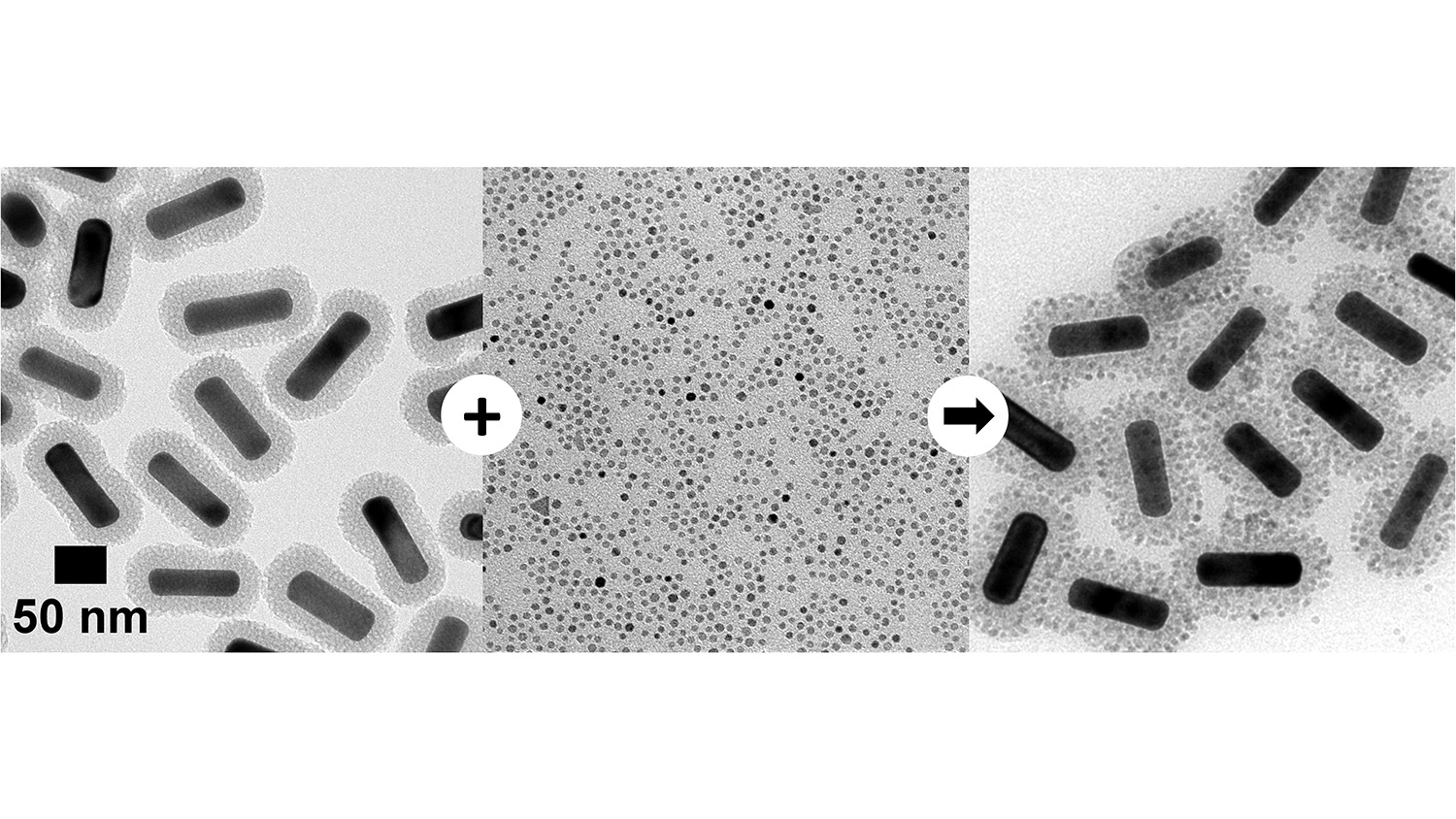
The new technique uses an approach called heteroaggregation. Silica-coated gold nanorods are dispersed in ethanol, a polar solvent. In ethanol, the hydrogen atoms are partially positively charged, and the oxygen atoms are partially negatively charged. The magnetite nanoparticles are dispersed in hexanes, a non-polar solvent, where the charges are not separated. When the two solutions are mixed, the magnetite nanoparticles bind to the gold nanorods – and the resulting magnetite-gold nanoparticles are removed from the solvent using a simple centrifugation process.
“We are able to take pre-synthesized, silica-coated gold nanorods and iron oxide nanoparticles and then combine them,” says Brian Chapman, a Ph.D. student at NC State and lead author of the paper. “This is simpler than other techniques, which rely on either growing iron oxide nanoparticles on gold nanorods or using molecular cross-linkers to bind the iron to the silica coating of the nanorods.”
“Our approach also results in highly uniform nanoparticles,” Tracy says. “And by incorporating ligands called PEG-catechols, the resulting nanoparticles can be dispersed in water. This makes them more useful for biomedical applications.
“These are interesting, and potentially very useful, multifunctional nanoparticles,” Tracy adds. “And hopefully this work will facilitate the development of applications that capitalize on them.”
The paper, “Heteroaggregation Approach for Depositing Magnetite Nanoparticles onto Silica-Overcoated Gold Nanorods,” is published in the journal Chemistry of Materials. The paper was co-authored by Wei-Chen Wu, a former Ph.D. student at NC State; and Qiaochu Li and Niels Holten-Andersen of MIT. The work was done with support from the National Science Foundation under grants DMR-1121107, DMR-1056653, and CBET-1605699.
-shipman-
Note to Editors: The study abstract follows.
“Heteroaggregation Approach for Depositing Magnetite Nanoparticles onto Silica-Overcoated Gold Nanorods”
Authors: Brian S. Chapman, Wei-Chen Wu and Joseph B. Tracy, North Carolina State University; Qiaochu Li and Niels Holten-Andersen, Massachusetts Institute of Technology
Published: Dec. 11, Chemistry of Materials
DOI: 10.1021/acs.chemmater.7b03481
Abstract: Hydrophobic, oleylamine-stabilized magnetite nanoparticles (Fe3O4 NPs) dispersed in hexanes can assemble into dense coatings on the surface of silica-overcoated gold nanorods (SiO2-GNRs) dispersed in ethanol by mixing. In this non-aqueous heteroaggregation process, Fe3O4 NPs are destabilized when ethanol is added, resulting in core/satellite Fe3O4-SiO2-GNRs within a few minutes. The composition of the solvent mixture allows tuning of the polarity and driving forces toward aggregation. At the optimal 2:1 volume ratio of hexanes:ethanol, heteroaggregation to form Fe3O4-SiO2-GNRs occurs quickly, while avoiding homoaggregation of Fe3O4 NPs or SiO2-GNRs. Fe3O4-SiO2-GNRs retain the longitudinal surface plasmon resonance of the gold nanorod cores and are magnetically responsive and separable. The Fe3O4 NPs remain bound on the surface of the Fe3O4-SiO2-GNRs during multiple cycles of magnetic extraction and redispersion. Oleylamine ligands on the Fe3O4 NPs render the Fe3O4-SiO2-GNRs dispersible in non-polar solvents. Functionalization of the outer Fe3O4 surface with poly(ethylene glycol) catechol (PEG-catechol) for PEGylation results in PEG-Fe3O4-SiO2-GNRs that disperse in water. In comparison with seeded growth or use of molecular crosslinkers to form multifunctional nanoparticles, heteroaggregation approaches are potentially quite general, simple, and efficient. The ability to continuously adjust the solvent polarity is expected to allow tuning of the heteroaggregation process for many different types and sizes of NPs.
- Categories: