Researchers Control Surface Tension to Manipulate Liquid Metals
For Immediate Release
Researchers from North Carolina State University have developed a technique for controlling the surface tension of liquid metals by applying very low voltages, opening the door to a new generation of reconfigurable electronic circuits, antennas and other technologies. The technique hinges on the fact that the oxide “skin” of the metal – which can be deposited or removed – acts as a surfactant, lowering the surface tension between the metal and the surrounding fluid.
The researchers used a liquid metal alloy of gallium and indium. In base, the bare alloy has a remarkably high surface tension of about 500 millinewtons (mN)/meter, which causes the metal to bead up into a spherical blob.
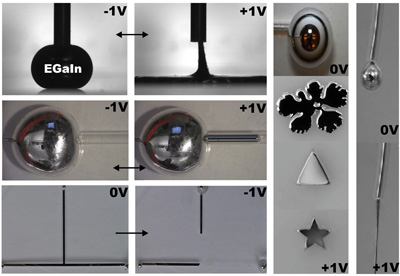
“But we discovered that applying a small, positive charge – less than 1 volt – causes an electrochemical reaction that creates an oxide layer on the surface of the metal, dramatically lowering the surface tension from 500 mN/meter to around 2 mN/meter,” says Dr. Michael Dickey, an associate professor of chemical and biomolecular engineering at NC State and senior author of a paper describing the work. “This change allows the liquid metal to spread out like a pancake, due to gravity.”
The researchers also showed that the change in surface tension is reversible. If researchers flip the polarity of the charge from positive to negative, the oxide is eliminated and high surface tension is restored. The surface tension can be tuned between these two extremes by varying the voltage in small steps.
“The resulting changes in surface tension are among the largest ever reported, which is remarkable considering it can be manipulated by less than one volt,” Dickey says. “We can use this technique to control the movement of liquid metals, allowing us to change the shape of antennas and complete or break circuits. It could also be used in microfluidic channels, MEMS, or photonic and optical devices. Many materials form surface oxides, so the work could extend beyond the liquid metals studied here.”
Dickey’s lab had previously demonstrated a technique for “3-D printing” liquid metals, which used the oxide layer formed in air to help the liquid metal retain its shape – the exact opposite of what the oxide layer does to the alloy in a basic solution.
“We think the oxide’s mechanical properties are different in a basic environment than they are in ambient air,” Dickey says.
The paper, “Giant and Switchable Surface Activity of Liquid Metal via Surface Oxidation,” was published online in the Proceedings of the National Academy of Sciences September 16. Lead authors of the paper are Mohammad Rashed Khan and Collin Eaker, Ph.D. students at NC State. The paper was co-authored by Dr. Edmond Bowden, a professor of chemistry at NC State.
The research was supported by National Science Foundation (NSF) CAREER grant number CMMI-0954321 and the Research Triangle NSF Materials Research Science and Engineering Center on Programmable Soft Matter grant number DMR-1121107.
-shipman-
Note to Editors: The study abstract follows.
“Giant and Switchable Surface Activity of Liquid Metal via Surface Oxidation”
Authors: Mohammad Rashed Khan, Collin B. Eaker, Edmond Bowden, and Michael D. Dickey, North Carolina State University
Published: Online Sept. 16 in Proceedings of the National Academy of Sciences
DOI: 10.1073/pnas.1412227111
Abstract: We present a new method to control the interfacial tension of a liquid alloy of gallium via electrochemical deposition (or removal) of the oxide layer on its surface. In sharp contrast with conventional surfactants, this method provides unprecedented lowering of surface tension (≈500 mJ/m2 to near zero) using very low voltage and the change is completely reversible. This dramatic change in the interfacial tension enables a variety of new electrohydrodynamic phenomena. The ability to manipulate the interfacial properties of the metal promises rich opportunities in shape-reconfigurable metallic components in electronic, electromagnetic, and microfluidic devices without the use of toxic mercury. This work suggests that the wetting properties of surface oxides—which are ubiquitous on most metals and semiconductors—are intrinsic ‘surfactants’. The inherent asymmetric nature of the surface coupled with the ability to actively manipulate its energetics are expected to have important applications in electrohydrodynamics, composites, and melt processing of oxide-forming materials.
- Categories:



