For Immediate Release
Researchers from North Carolina State University, Duke University and Brookhaven National Laboratory have found that molybdenum sulfide (MoS2) holds more promise than previously thought as a catalyst for producing hydrogen to use as a clean energy source. Specifically, the researchers found that the entire surface of MoS2 can be used as a catalyst, not just the edges of the material.
“The key finding here is that the intrinsic catalytic performance of MoS2 is much better than the research community thought,” says Linyou Cao, an associate professor of materials science and engineering at NC State and senior author of a paper describing the work. “We’re optimistic that this can be a step toward making hydrogen a larger part of our energy portfolio.”
Hydrogen promises clean energy, producing only water as a byproduct. But to create hydrogen for use as a clean energy source, ideally you’d be able to isolate the hydrogen gas from water – with the only byproduct being oxygen.
However, the key to creating hydrogen from water – a process called hydrogen evolution – is an efficient catalyst. Currently, the best catalyst is platinum, which is too expensive for widespread use.
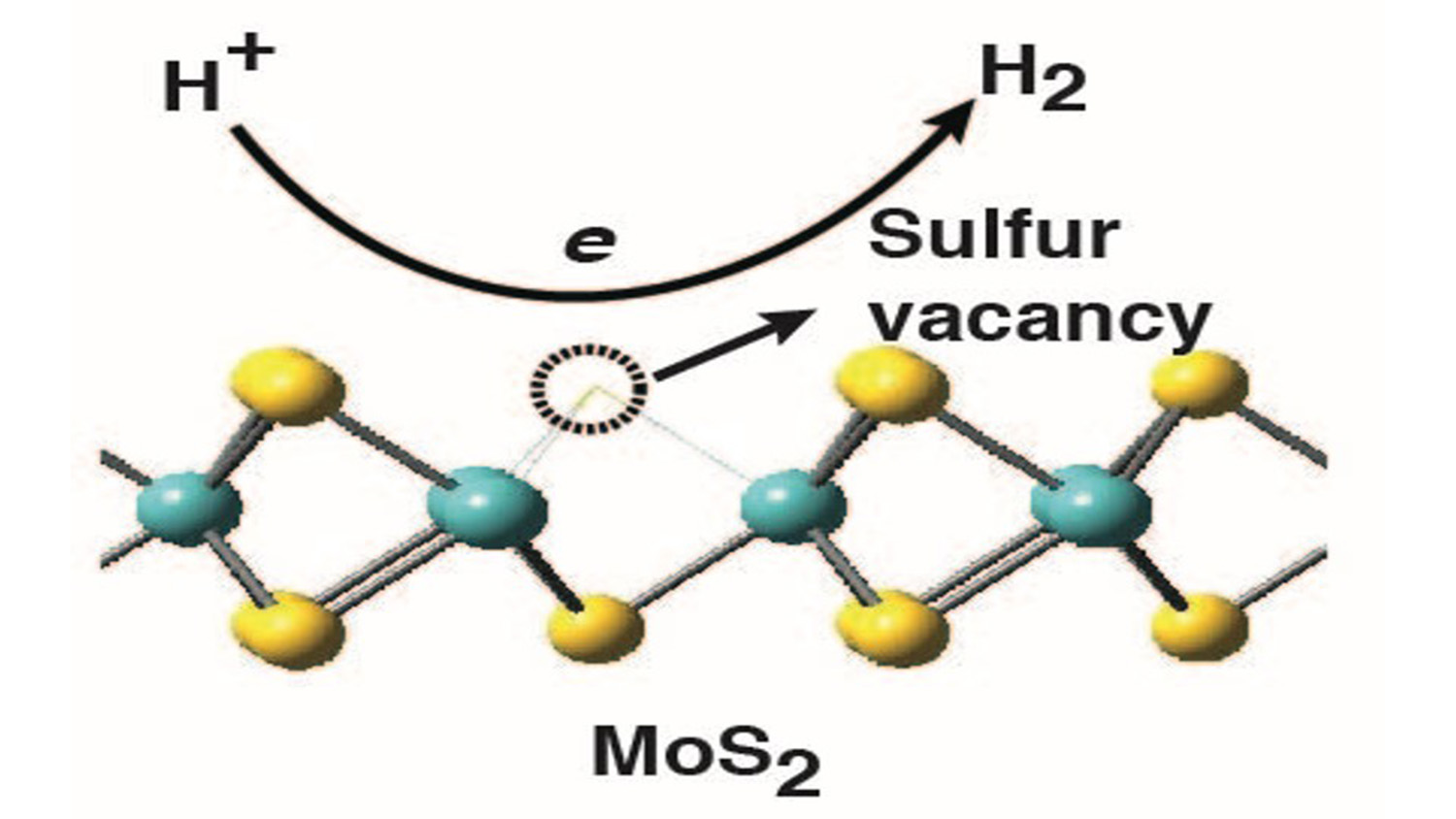
Another candidate for a hydrogen evolution catalyst is MoS2, which is both inexpensive and abundant. But it has long been thought that MoS2 is of limited utility, based on the conventional wisdom that only the edges of MoS2 act as catalysts – leaving the bulk of the material inactive.
But the new findings from NC State, Duke and Brookhaven show that the surface of MoS2 can be engineered to maximize the catalytic efficiency of the material. And the key to this efficiency is the number of sulfur vacancies in the MoS2.
If you think of the crystalline structure of MoS2 as a grid of regularly spaced molybdenum and sulfur atoms, a sulfur vacancy is what happens when one of those sulfur atoms is missing.
“We found that these sulfur vacancies attract the hydrogen atoms in water at just the right strength: the attraction is strong enough pull the hydrogen out of the water molecule, but is then weak enough to let the hydrogen go,” says Cao.
The researchers also found that the grain boundaries of MoS2 , which have been speculated by the research community to be catalytically active for hydrogen evolution, may only provide trivial activity. Grain boundaries are the boundaries between crystalline domains.
The findings point to a new direction for improving the catalytic performance of MoS2 . Currently, the most common way is to increase the number of edge sites, because of the conventional wisdom that only the edge sites are catalytically active.
“Our result indicates that grain boundaries should not be the factor to consider when thinking about improving catalytic activity,” Cao says. “The best way to improve the catalytic activities is to engineer sulfur vacancies. The edges of MoS2 are still twice as efficient at removing hydrogen atoms compared to the sulfur vacancies. But it’s difficult to create a high density of edges in MoS2 – a lot of the material’s area is wasted – whereas a large number of sulfur vacancies can be engineered uniformly across the material.”
The researchers have also found that there is a “sweet spot” for maximizing the catalytic efficiency of MoS2 .
“We get the best results when between 7 and 10 percent of the sulfur sites in MoS2 are vacant,” Cao says. “If you go higher or lower than that range, catalytic efficiency drops off significantly.”
Additionally, the researchers found that the crystalline quality of MoS2 is important to optimize the catalytic activity of the sulfur vacancies. The sulfur vacancies in high crystalline quality MoS2 showed better efficiency than those in low crystalline quality MoS2 , even when the densities of the vacancies are the same.
“In order to get the best output from sulfur vacancies, the crystalline quality of MoS2 needs to be very high,” says Guoqing Li, a Ph.D. student at NC State and lead author of the paper. “The ideal scenario would be 7 to 10 percent sulfur vacancies uniformly distributed in a single crystalline MoS2 film.”
The work was done using MoS2 thin films that are only three atoms thick. Using these engineered thin films, the researchers were able to achieve catalytic efficiency comparable to previous MoS2 technologies that relied on having two or three orders of magnitude more surface area.
“We now know that MoS2 is a more promising catalyst than we anticipated, and are fine-tuning additional techniques to further improve its efficiency,” Cao says. “Hopefully, this moves us closer to making a low-cost catalyst that is at least as good as platinum.”
The paper, “All the Catalytic Active Sites of MoS2 for Hydrogen Evolution,” is published in the Journal of the American Chemical Society. The paper was co-authored by Yifei Yu, David Peterson, Abdullah Zafar, Raj Kumar, Frank Hunte and Steve Shannon of NC State; Du Zhang, Stefano Curtarolo and Weitao Yang of Duke; and Qiao Qiao and Yimei Zhu of Brookhaven National Lab.
The work was done with support from the Department of Energy’s Office of Science, under grants DE-SC0012575 and DE-SC0012704, as well as by the National Science Foundation under grant PHY1338917.
-shipman-
Note to Editors: The study abstract follows.
“All the Catalytic Active Sites of MoS2 for Hydrogen Evolution”
Authors: Guoqing Li, Yifei Yu, David Peterson, Abdullah Zafar, Raj Kumar, Frank Hunte, Steve Shannon and Linyou Cao, North Carolina State University; Du Zhang, Stefano Curtarolo and Weitao Yang, Duke University; Qiao Qiao and Yimei Zhu, Brookhaven National Laboratory
Published: online Nov. 29, Journal of the American Chemical Society
DOI: 10.1021/jacs.6b05940
Abstract: MoS2 presents a promising low-cost catalyst for the hydrogen evolution reaction (HER), but the understanding about its active sites has remained to be limited. Here we present an unambiguous study for the catalytic activities of all possible reaction sites of MoS2, including edge sites, sulfur vacancies, and grain boundaries. We demonstrate that, in addition to the well-known catalytically active edge sites, sulfur vacancies provide another major active site for the HER while the catalytic activity of grain boundaries is much weaker. The intrinsic turnover frequencies (Tafel slopes) of the edge sites, sulfur vacancies, and grain boundaries are estimated to be 7.5 s-1 (65-75 mV/dec), 3.2 s-1 (65-85 mV/dec), and 0.1 s-1 (120-160 mV/dec), respectively. We also demonstrate that the catalytic activity of sulfur vacancies strongly depends on the density of the vacancies and the local crystalline structure at the proximity of the vacancies. Unlike edge sites, whose catalytic activity linearly depends on the length, sulfur vacancies show optimal catalytic activities when the vacancy density is in the range of 7-10%. And the sulfur vacancies in high crystalline quality MoS2 is higher than those in low crystalline quality MoS2, which may be related with different local crystalline structures at the proximity of the vacancies.
- Categories: