No Pollen, No Seeds: Genome Editing Technique Yields Useful Traits

For Immediate Release
North Carolina State University researchers have successfully transferred an important gene from one compartment of a plant cell to another to produce tobacco plants that lack pollen and viable seeds, while otherwise growing normally. Their findings could lead to better ways of producing hybrid seeds to maximize crop productivity, or to introduce seedlessness in fruit species lacking the often-desired trait, such as raspberries, blackberries or muscadine grapes.
The researchers began the work in the energy-producing portion of a cell, the mitochondria. In plants, aberrations within the mitochondrial genome can be associated with the inability to make pollen, a trait known as cytoplasmic male sterility (CMS) that has been successfully exploited for the production of high-yielding hybrid seeds in many important crops. Naturally occurring CMS-based systems that are robust enough to facilitate commercial scale hybrid seed production are limited, however.
In their proof-of-concept study, the NC State researchers, together with colleagues from Precision BioSciences and Elo Life Systems, deployed a unique strategy to test whether the CMS trait could be generated in tobacco, a commonly used model species in plant research. The researchers initially took an essential mitochondrial gene called atp1 and moved it to the nucleus after placing it under the regulatory control of an element – known as a promoter – that they predicted would allow the transferred atp1 gene to be expressed in every cell of the plant except those responsible for producing pollen. The researchers then used genome editing tools to permanently remove the native atp1 gene from the mitochondria.
Their approach was successful.
“The results exceeded our expectations,” said Ralph Dewey, Philip Morris Professor of Crop Science at NC State and corresponding author of a paper describing the research. “The plants looked completely normal up until they began to flower, but then failed to produce pollen because the transferred atp1 gene was no longer expressed. Importantly, because the original atp1 gene was deleted from the mitochondrial genome, the trait will be inherited maternally, which is a crucial consideration for large-scale hybrid seed production.”
Pollen wasn’t the only casualty of the technique. When cross-fertilized using pollen from a neighboring normal plant, their tobacco plants unexpectedly produced small, hollow seeds, much like those seen in popular “seedless” fruits such as watermelons and grapes.
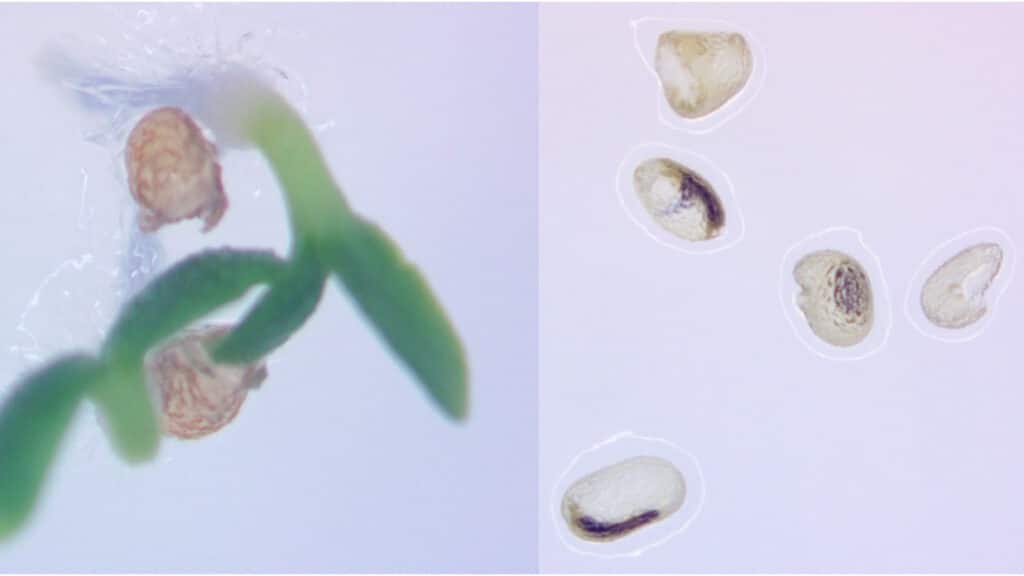
“That’s because the promoter that we chose not only failed to express during pollen formation, but also during early seed development,” Dewey said.
Dewey said that his team is now working to decouple these results so that researchers can achieve either pollen infertility or the seedless trait alone, instead of both at the same time.
Dewey also stressed that the findings shouldn’t be limited to tobacco plants. Their next generation of experiments will include testing the seedless trait in tomato, a close relative of tobacco. They will also test their novel CMS trait in a grain such as rice to test the efficacy of their system in a crop where hybrid seed production is important for achieving maximal yields.
“Knowing the way the system works, there’s no reason to believe that we couldn’t effectively transfer the technology to other plant species,” he said.
The study appears in Frontiers in Plant Science. Devarshi Selote, H. Carol Griffin, Allison N. Dickey, Derek Jantz, J. Jeff Smith, Anna Matthiadis, Josh Strable, Caitlin Kestell and William A. Smith co-authored the paper. The work was funded in part by a grant from Elo Life Systems and supported by the NC State Plant Breeding Consortium. Dewey and several other researchers have filed a patent on the new technique.
-kulikowski-
Note to editors: The abstract of the paper follows.
“Cytoplasmic Male Sterility and Abortive Seed Traits Generated through Mitochondrial Genome Editing Coupled with Allotopic Expression of atp1 in Tobacco”
Authors: Ralph E. Dewey, Devarshi Selote, H. Carol Griffin, Allison N. Dickey, Derek Jantz, J. Jeff Smith, Anna Matthiadis, Josh Strable, Caitlin Kestell, William A. Smith
Published: Sept. 15, 2023 in Frontiers in Plant Science
DOI: 10.3389/fpls.2023.1253640
Abstract: Allotopic expression is the term given for the deliberate relocation of gene function from an organellar genome to the nuclear genome. We hypothesized that the allotopic expression of an essential mitochondrial gene using a promoter that expressed efficiently in all cell types except those responsible for male reproduction would yield a cytoplasmic male sterility (CMS) phenotype once the endogenous mitochondrial gene was inactivated via genome editing. To test this, we repurposed the mitochondrially encoded atp1 gene of tobacco to function in the nucleus under the transcriptional control of a CaMV 35S promoter (construct 35S:nATP1), a promoter that has been shown to be minimally expressed in early stages of anther development. The endogenous atp1 gene was eliminated (delta atp1) from 35S:nATP1 tobacco plants using custom-designed meganucleases directed to the mitochondria. Vegetative growth of most 35S:nATP1/delta atp1 plants appeared normal, but upon flowering produced malformed anthers that failed to shed pollen. When 35S:nATP1/delta atp1 plants were cross-pollinated, ovary/capsule development appeared normal, but the vast majority of the resultant seeds were small, largely hollow and failed to germinate, a phenotype akin to the seedless trait known as stenospermocarpy. Characterization of the mitochondrial genomes from three independent delta atp1 events suggested that spontaneous recombination over regions of microhomology and substoichiometric shifting were the mechanisms responsible for atp1 elimination and genome rearrangement in response to exposure to the atp1-targeting meganucleases. Should the results reported here in tobacco prove to be translatable to other crop species, then multiple applications of allotropic expression of an essential mitochondrial gene followed by its elimination through genome editing can be envisaged. Depending on the promoter(s) used to drive the allotopic gene, this technology may have potential application in the areas of: (1) CMS trait development for use in hybrid seed production; (2) seedless fruit production; and (3) transgene containment.


