Recreating the Web of Blood Vessels That Keep Human Tissue Alive

For years, one of the largest obstacles facing the field of regenerative medicine – the science of growing new human parts from scratch – was the need to create a circulatory system to support new tissues and organs as they grew. Now two researchers from North Carolina State University and the University of North Carolina at Chapel Hill are being recognized for creating technology to make the customized blood vessels necessary to support tissue generation.
Your blood vessels are amazing. Altogether, the average adult has about 60,000 miles of blood vessels in his or her body. These vessels – and the blood they move – are absolutely essential. Without the oxygen and nutrients they provide, your organs and tissues would die. Without its effective (and efficient) ability to transfer substances throughout the body, your immune system wouldn’t work. Basically: no blood vessels, no life.
The smallest of your blood vessels – your microvasculature – are particularly important. They account for about 50,000 miles of your circulatory system. They’re everywhere. And they have, historically, been very, very difficult to recreate.
This is where Frances Ligler and Michael Daniele come in.
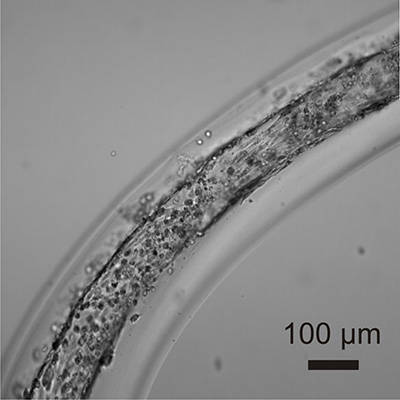
In the early 2000s, while a researcher at the U.S. Naval Research Laboratory (NRL), Ligler developed and patented devices and a technique for creating and shaping continuous fibers on the micron scale. For frame of reference, a single hair on your head is between 40 and 50 microns – or micrometers – wide. Ligler’s advance allows users to create the fibers out of any polymer that can be made quickly. But that’s not all.
“You can envision any structure you want, such as multi-layered hollow tubes, and modify the devices to produce that structure efficiently and continuously,” says Ligler, now the Lampe Distinguished Professor in the joint Biomedical Engineering Department at NC State and UNC-Chapel Hill.
And that patented technology led to a new question: if Ligler’s advance could be used to make customized hollow tubes on the micron scale, could it be used to create microvasculature?
Ligler and other researchers at NRL – including a fellow engineer named Michael Daniele – decided to find out.
The first step was to identify a combination of polymers that had two particular characteristics: they needed to be compatible with the continuous fiber manufacturing process; and they also needed to be not only compatible with human cells, but actually encourage cell growth and function.
Once Daniele identified the correct materials, the researchers began creating multi-layered microvessels. Each layer of these microvessels is designed to perform a different function, just like the real blood vessels in the human body.
The fabrication process itself is a major breakthrough. Human cells of a specific type can be mixed with appropriately customized materials destined for each layer of the microvessel. All mixtures are then focused simultaneously into the appropriate layers as the microvessels are built.
The highly complex structure looks, generally, like this: at the center you have a hollow tube, whose walls are lined with the endothelial cells that handle everything from removing waste from the surrounding tissue to transferring oxygen and nutrients into and out of the blood; the next layer consists of a polymer embedded with smooth muscle cells that make the blood vessel flexible without losing its shape; the third – and final – layer is a polymer embedded with cells that produce fibrous material to physically support the blood vessel.
“These three layers are what you’d expect to see in a major blood vessel, like the femoral artery in your leg,” says Daniele, now an assistant professor in NC State’s Department of Electrical and Computer Engineering as well as the joint Biomedical Engineering Department. “But our technique can be modified; you don’t have make all three layers. This is important, because the tiniest blood vessels in our bodies – like the capillaries in your extremities – are lined only with endothelial cells.”
These two advances are being recognized by NRL at an award ceremony on March 18. Daniele and Ligler will receive an Edison Award from NRL for their technique for creating blood vessels. Ligler will receive an additional Edison Award for her work enabling the creation and shaping of continuous fibers on the micron scale. The highly competitive Edison Award goes to NRL researchers who have been issued a patent for advances judged to be of significant industry interest or potential impact on Department of Defense operations.
These two advances are already forming the foundation for a host of ongoing research initiatives, including many at NC State and UNC-Chapel Hill:
- Ligler is working with Ke Cheng to put microvasculature into cardiac stem cells in order to regenerate heart tissue. “We want to know how cardiac stem cells fix hearts that have been damaged by heart attacks,” Ligler explains.
- Ligler is also working with Jaqueline Cole and Elizabeth Loboa to put microvasculature into place in stem cells that generate cartilage and bone. Loboa, a former researcher at NC State and UNC-Chapel Hill, is now dean of the College of Engineering at the University of Missouri.
- Daniele is working on a project that would utilize the microvasculature technology to create three-dimensional biological structures that mimic the blood-brain barrier found in humans. “If we can better understand the blood-brain barrier, we will be better able to develop and deliver treatments that address strokes, brain cancer and other medical problems that currently require us to access the brain directly through the skull,” Daniele says.
- Daniele is also working with Stefano Menegatti, Wolfgang Baeumer, and NRL’s Kyle DeVito to use the microvascular technology to help create a full in vitro model of human skin. “Creating a model like this one could help us understand a host of issues related to how chemicals or biological agents pass through the skin and enter the bloodstream,” Daniele says.
“The microvessel technology solves a critical hurdle for creating new tissues and provides an important tool for evaluating new therapies,” Ligler says. “The availability of microvessels that can be produced in continuous mode and easily manipulated complements NC State’s existing programs in bioprinting and tissue regeneration from stem cells and should make NC State an international leader in three-dimensional tissue engineering.”
- Categories:


