New Technique for Creating NV-Doped Nanodiamonds May Be Boost for Quantum Computing
For Immediate Release
Researchers at North Carolina State University have developed a new technique for creating NV-doped single-crystal nanodiamonds, only four to eight nanometers wide, which could serve as components in room-temperature quantum computing technologies. These doped nanodiamonds also hold promise for use in single-photon sensors and nontoxic, fluorescent biomarkers.
Currently, computers use binary logic, in which each binary unit – or bit – is in one of two states: 1 or 0. Quantum computing makes use of superposition and entanglement, allowing the creation of quantum bits – or qubits – which can have a vast number of possible states. Quantum computing has the potential to significantly increase computing power and speed.
A number of options have been explored for creating quantum computing systems, including the use of diamonds that have “nitrogen-vacancy” centers. That’s where this research comes in.
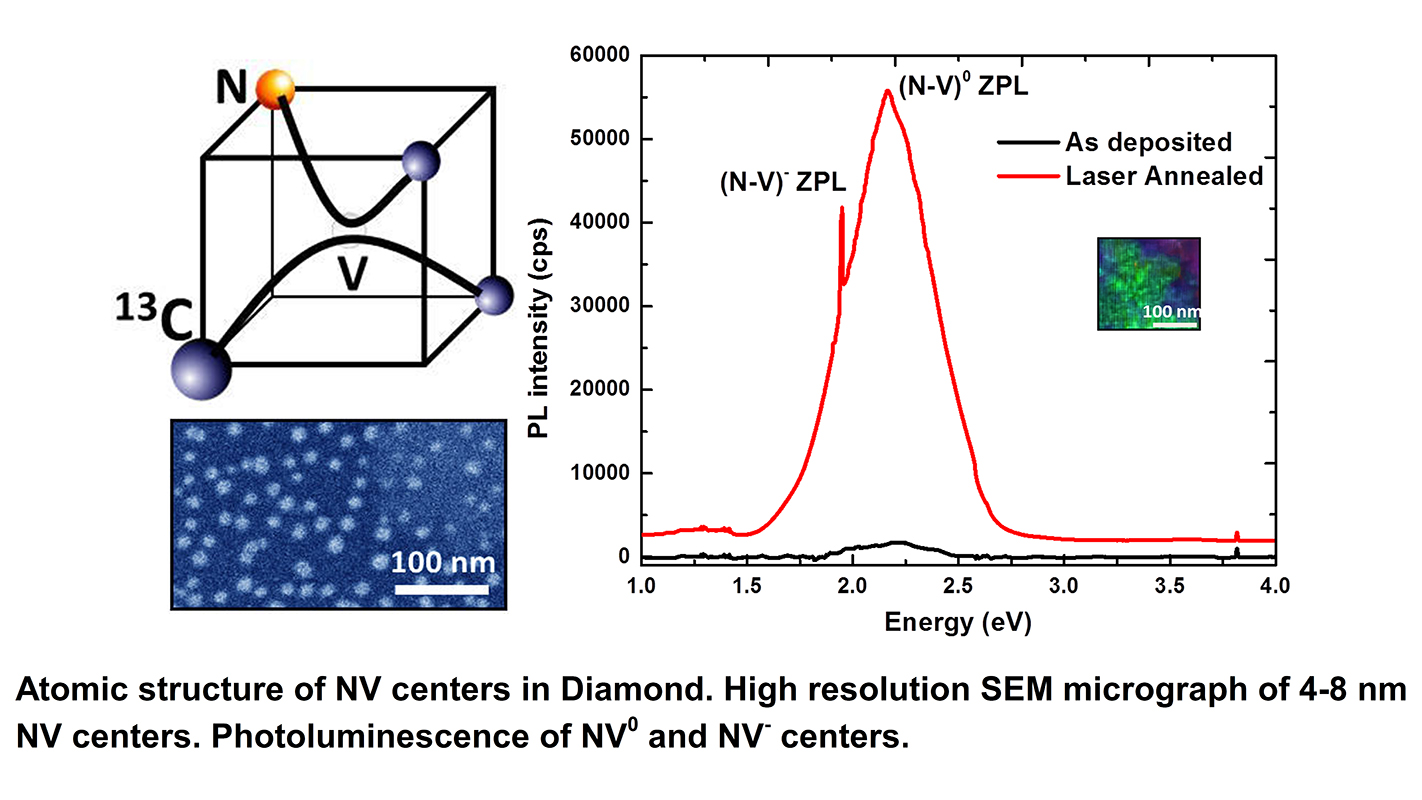
Normally, diamond has a very specific crystalline structure, consisting of repeated diamond tetrahedrons, or cubes. Each cube contains five carbon atoms. The NC State research team has developed a new technique for creating diamond tetrahedrons that have two carbon atoms; one vacancy, where an atom is missing; one carbon-13 atom (a stable carbon isotope that has six protons and seven neutrons); and one nitrogen atom. This is called the NV center. Each NV-doped nanodiamond contains thousands of atoms, but has only one NV center; the remainder of the tetrahedrons in the nanodiamond are made solely of carbon.
It’s an atomically small distinction, but it makes a big difference.
“That little dot, the NV center, turns the nanodiamond into a qubit,” says Jay Narayan, the John C. Fan Distinguished Chair Professor of Materials Science and Engineering at NC State and lead author of a paper describing the work. “Each NV center has two transitions: NV0 and NV–. We can go back and forth between these two states using electric current or laser. These nanodiamonds could serve as the basic building blocks of a quantum computer.”
To create these NV-doped nanodiamonds, the researchers start with a substrate, such as such as sapphire, glass or a plastic polymer. The substrate is then coated with amorphous carbon – elemental carbon that, unlike graphite or diamond, does not have a regular, well-defined crystalline structure. While depositing the film of amorphous carbon, the researchers bombard it with nitrogen ions and carbon-13 ions. The carbon is then hit with a laser pulse that raises the temperature of the carbon to approximately 4,000 Kelvin (or around 3,727 degrees Celsius) and is then rapidly quenched. The operation is completed within a millionth of a second and takes place at one atmosphere – the same pressure as the surrounding air. By using different substrates and changing the duration of the laser pulse, the researchers can control how quickly the carbon cools, which allows them to create the nanodiamond structures.
“Our approach reduces impurities; controls the size of the NV-doped nanodiamond; allows us to place the nanodiamonds with a fair amount of precision; and directly incorporates carbon-13 into the material, which is necessary for creating the entanglement required in quantum computing,” Narayan says. “All of the nanodiamonds are exactly aligned through the paradigm of domain matching epitaxy, which is a significant advance over existing techniques for creating NV-doped nanodiamonds.”
“The new technique not only offers unprecedented control and uniformity in the NV-doped nanodiamonds, it is also less expensive than existing techniques,” Narayan says. “Hopefully, this will enable significant advances in the field of quantum computing.”
The researchers are currently talking with government and private sector groups about how to move forward. One area of interest is to develop a means of creating self-assembling systems that incorporate entangled NV-doped nanodiamonds for quantum computing.
The paper, “Novel synthesis and properties of pure and NV-doped nanodiamonds and other nanostructures,” is published in the journal Materials Research Letters. The paper was co-authored by Anagh Bhaumik, a Ph.D. student at NC State. The work was supported by the U.S. Army Research Office under grant W911NF-12-R-0012-03.
-shipman-
Note to Editors: The study abstract follows.
“Novel synthesis and properties of pure and NV-doped nanodiamonds and other nanostructures”
Authors: Jagdish Narayan and Anagh Bhaumik, North Carolina State University
Published: Nov. 2, Materials Research Letters
DOI: 10.1080/21663831.2016.1249805
Abstract: We report a novel method for synthesis and processing of pure and nitrogen-vacancy (NV)-doped nanodiamonds with sharp NV0 and NV– transitions at ambient temperatures and pressures in air. Carbon films are melted by nanosecond lasers in super undercooled state and quenched rapidly. We can form single-crystal nanodiamonds, microdiamonds, nanoneedles and microneedles, and large area films. Substitutional nitrogen atoms and vacancies are incorporated into diamond during rapid liquid-phase growth, where dopant concentrations can far exceed thermodynamic solubility limits through solute trapping. These nanodiamonds can be placed deterministically and the transitions between NV– and NV0 can be controlled electrically and optically by laser illumination.
- Categories:


