Molecular Motors and Molecular Machines – Q&A With Gufeng Wang
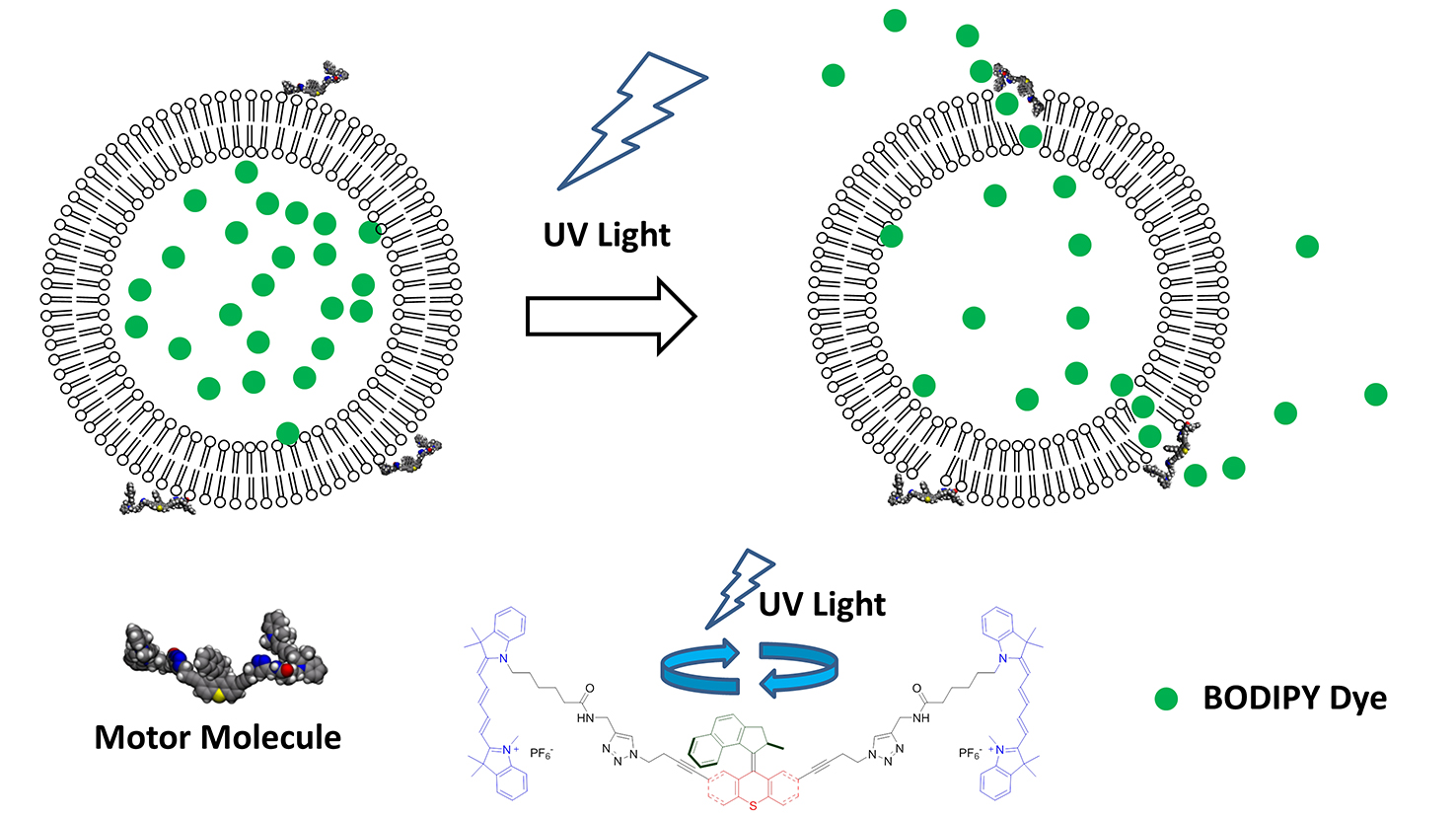
Chemist Gufeng Wang was part of a team of university researchers from Rice, Durham (U.K.) and NC State that created light-driven, motorized molecules capable of drilling holes in the membranes of individual cells. These molecular machines show promise for either bringing therapeutic agents (drugs) into the cells or directly inducing the cells to die. The researchers’ work appears in Nature. Wang sat down with the Abstract to answer some questions about the project.
The Abstract (TA): The team essentially created a molecular “motor” that can drill through the cell membrane once it physically attaches to specific types of cells. What are some of the uses for this technology?
Wang: People usually open up cell membranes for two purposes: to deliver drugs (for medication) or genes (treating diseases at the genetic level). The usual ways to open up a cell membrane include using mechanical force (particle bombardment), electric charge or chemical perforation. These methods don’t have specificity; in other words, they don’t let you differentiate between normal and diseased cells. Or, you can use targeted drug delivery, in which you load the delivery vehicle (such as a vesicle or nanoparticle) with drugs, and modify the vehicle with a molecular recognition component (like an antibody) that specifically targets a receptor on the cell membrane. Then, you wait for the cells to engulf the vehicle or the drug naturally through endocytosis. As you might imagine, this method isn’t very efficient, although it does reduce the amount of the drug used and the side effects of the drug on normal cells.
In our approach, we use a motor attached to a molecular recognition component – a peptide in this case – as the delivery vehicle. This allows it to bind to specific locations and open the membrane at a designated location on cells. It is also controllable because it uses light to trigger the opening event. So it has advantages over both methods mentioned above.
This technology can be used as a highly efficient, highly specific drug delivery method or as a way to kill cells directly by disrupting their material exchange with the environment. Skin cancer is one condition that might be treated by simply killing the malignant cells.
TA: The motors used ultraviolet (UV) light as the power source. In the absence of that light, would the motorized molecules merely drift through the body, or do they still move toward the targeted cells? Can UV light penetrate deeply enough to be used with this technology in humans?
Wang: Without the activation of the motor, the complex (motor and peptide) still binds to the cell surface. But it doesn’t do any damage to the cell membrane.
With current UV activation, we can only treat cells at the surface of the tissue, for example, skin or gastrointestinal mucosa, where the light can be directed through a fiber optic. This is because the UV has very shallow penetration depth. In the future, using motors that can be activated using near infra-red (IR) light, this limit can be removed.
TA: What does the future hold for this work?
Wang: Because of the limitation of the UV light (current motor can only be activated by UV photons that have enough energy to move the rotor), we are working on two directions to make this approach more bio-friendly. The first is that we (mainly researchers at Rice) are developing visible light or even near IR light activated motors. The second is that we are trying to activate the same motor using near IR light through a process called multiphoton excitation (MPE). In this process, the motor will absorb two photons simultaneously and get enough energy to start the rotor. Since near IR light has deep penetration depth, we are no longer limited to the surface of the tissue. Again, we are looking for biomedical and bioengineering applications for this technique.
- Categories: