Off-the-Shelf Artificial Cardiac Patch Repairs Heart Attack Damage in Rats, Pigs
For Immediate Release
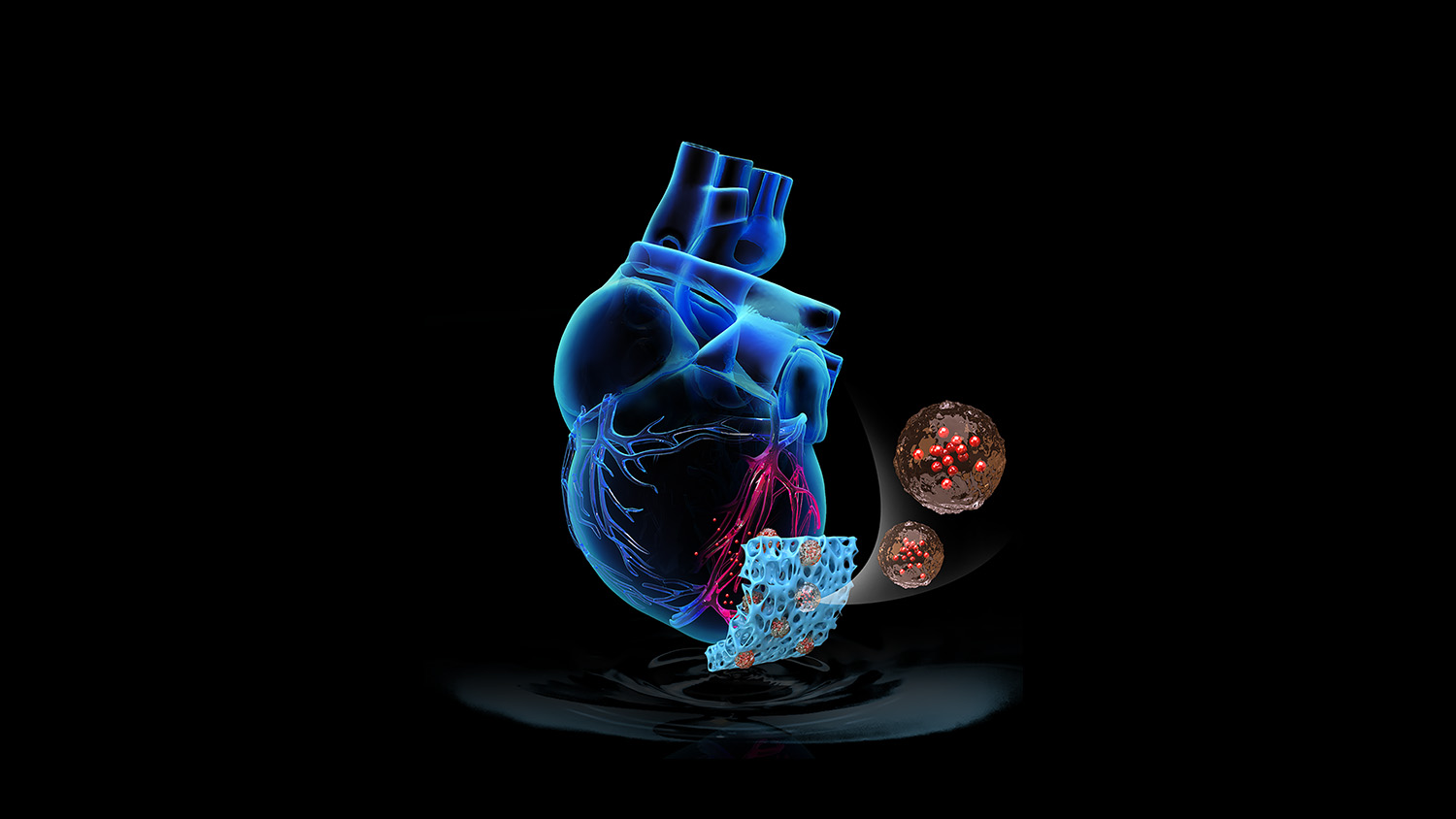
Researchers from North Carolina State University have developed an “off-the-shelf” artificial cardiac patch that can deliver cardiac cell-derived healing factors directly to the site of heart attack injury. In a rat model of heart attack, the freezable, cell-free patch improved recovery. The researchers also found similar effects in a pilot study involving a pig model of heart attack.
Cardiac patches are being studied as a promising future option for delivering cell therapy directly to the site of heart attack injury. However, current cardiac patches are fragile, costly, time-consuming to prepare and, since they use live cellular material, increase risks of tumor formation and arrhythmia.
“We have developed an artificial cardiac patch that can potentially solve the problems associated with using live cells, yet still deliver effective cell therapy to the site of injury,” says Ke Cheng, Randall B. Terry, Jr. Distinguished Professor in Regenerative Medicine at NC State’s College of Veterinary Medicine and professor in the NC State/UNC Joint Department of Biomedical Engineering.
Cheng and colleagues from NC State and UNC-Chapel Hill built the patch by first creating a scaffolding matrix from decellularized pig cardiac tissue. Synthetic cardiac stromal cells – made of a biodegradable polymer containing cardiac stromal cell-derived repair factors – were embedded in the matrix. The resulting patch contained all of the therapeutics secreted by the cells, without live cells that could trigger a patient’s immune response.
In a rat model of heart attack, treatment with the artificial cardiac patch resulted in ~50% improvement of cardiac function over a three-week period compared to non-treatment, as well as a ~30% reduction in scarring at the injury site.
The researchers also conducted a seven-day pilot study of heart attack in a pig model, and saw ~30% reduction in scarring in some regions of the pig hearts, as well as stabilized heart function, compared to non-treatment.
Additional experiments demonstrated that artificial patches that had been frozen were equally potent to freshly created patches.
“The patch can be frozen and safely stored for at least 30 days, and since there are no live cells involved, it will not trigger a patient’s immune system to reject it,” Cheng says. “It is a first step toward a truly off-the-shelf solution to cardiac patch therapy.”
The research appears in Science Translational Medicine, and was funded by the National Institutes of Health and the American Heart Association. Cheng is corresponding author. Ke Huang, formerly a graduate student at NC State and currently a postdoctoral scientist at Duke University, is first author.
-peake-
Note to editors: An abstract follows.
“An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs”
DOI: 10.1126/scitranslmed.aat9683
Authors: Ke Huang, Teng Su, Junnan Tang, Deliang Shen, Li Qiao, Shiqi Hu, Zhenhua Li, Hongxia Liang, Kyle Mathews, Valery Scharf, Ke Cheng, North Carolina State University; Emily Ozpinar, Teng Su, Shiqi Hu, Zhenhua Li, Donald Freytes, Ke Cheng, NC State/UNC-Chapel Hill Joint Department of Biomedical Engineering
Published: April 8, 2020 in Science Translational Medicine
Abstract:
Cell therapy has been a promising strategy for cardiac repair after injury or
infarction; however, low retention and engraftment of transplanted cells limit
potential therapeutic efficacy. Seeding scaffold material with cells to create
cardiac patches that are transplanted onto the surface of the heart can
overcome these limitations. However, because patches need to be freshly
prepared to maintain cell viability, long-term storage is not feasible and
limits clinical applicability. Here, we developed an off-the-shelf therapeutic
cardiac patch composed of a decellularized porcine myocardial extracellular
matrix scaffold and synthetic cardiac stromal cells (synCSCs) generated by
encapsulating secreted factors from isolated human cardiac stromal cells. This
fully acellular artificial cardiac patch (artCP) maintained its potency after
long-term cryopreservation. In a rat model of acute myocardial infarction,
transplantation of the artCP supported cardiac recovery by reducing scarring,
promoting angiomyogenesis, and boosting cardiac function. The safety and
efficacy of the artCP were further confirmed in a porcine model of myocardial
infarction. The artCP is a clinically feasible, easy-to-store, and cell-free
alternative to myocardial repair using cell-based cardiac patches.
- Categories:


