For Immediate Release
Engineering researchers have developed a new technique for eliminating particularly tough blood clots, using engineered nanodroplets and an ultrasound “drill” to break up the clots from the inside out. The technique has not yet gone through clinical testing. In vitro testing has shown promising results.
Specifically, the new approach is designed to treat retracted blood clots, which form over extended periods of time and are especially dense. These clots are particularly difficult to treat because they are less porous than other clots, making it hard for drugs that dissolve blood clots to penetrate into the clot.
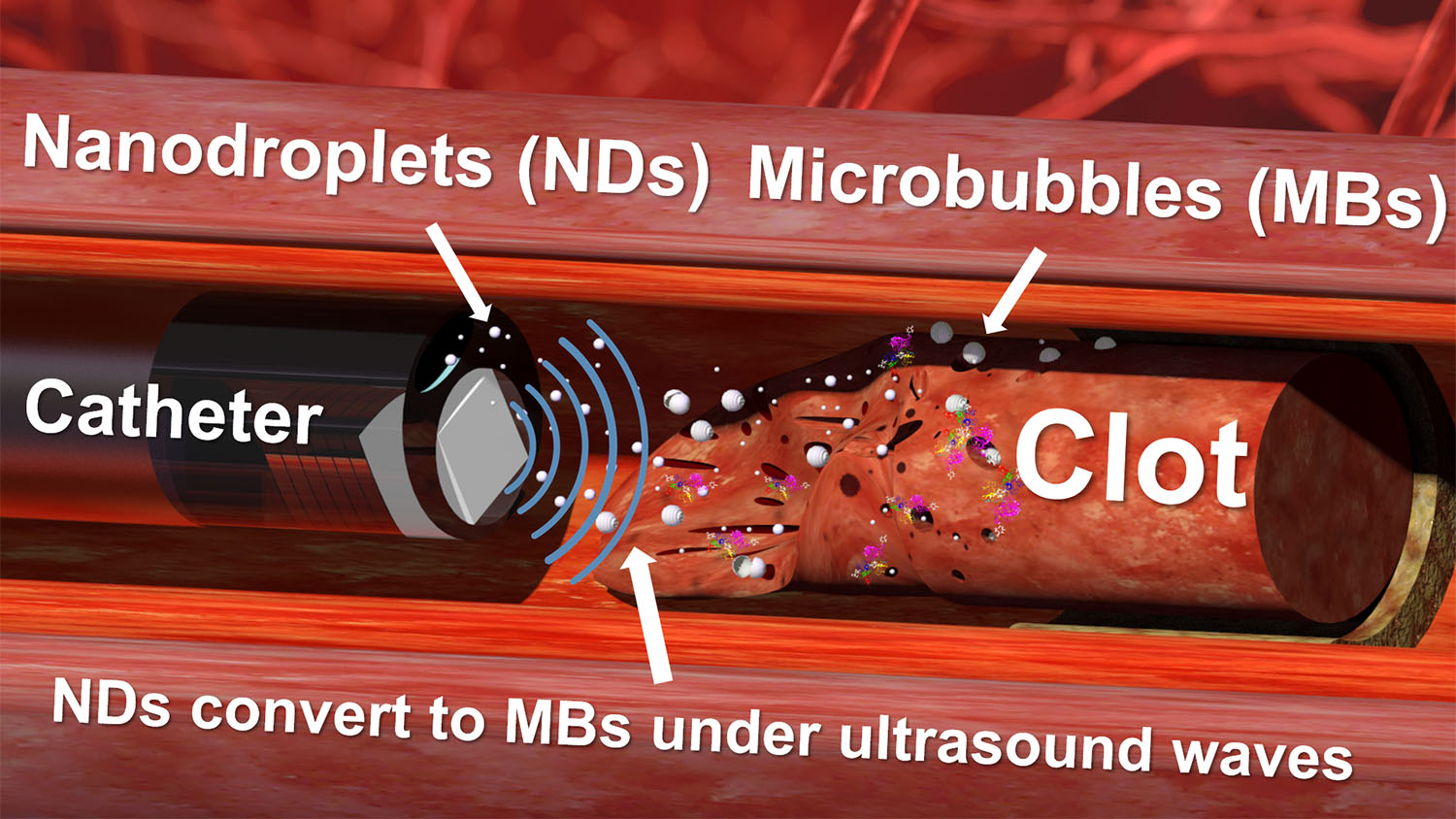
The new technique has two key components: the nanodroplets and the ultrasound drill.
The nanodroplets consist of tiny lipid spheres that are filled with liquid perfluorocarbons (PFCs). Specifically, the nanodroplets are filled with low-boiling-point PFCs, which means that a small amount of ultrasound energy will cause the liquid to convert into gas. As they convert into a gas, the PFCs expand rapidly, vaporizing the nanodroplets and forming microscopic bubbles.
“We introduce nanodroplets to the site of the clot, and because the nanodroplets are so small, they are able to penetrate and convert to microbubbles within the clots when they are exposed to ultrasound,” says Leela Goel, first author of a paper on the work. Goel is a Ph.D. student in the joint biomedical engineering department at North Carolina State University and the University of North Carolina at Chapel Hill.
After the microbubbles form within the clots, the continued exposure of the clots to ultrasound oscillates the microbubbles. The rapid vibration of the microbubbles causes them to behave like tiny jackhammers, disrupting the clot’s physical structure, and helping to dissolve the clots. This vibration also creates larger holes in the clot mass that allow blood borne anti-clotting drugs to penetrate deep into the clot and further break it down.
The technique is made possible by the ultrasound drill – which is an ultrasound transducer that is small enough to be introduced to the blood vessel via a catheter. The drill can aim ultrasound directly ahead, which makes it extremely precise. It is also able to direct enough ultrasound energy to the targeted location to activate the nanodroplets, without causing damage to surrounding healthy tissue. The drill incorporates a tube that allows users to inject nanodroplets at the site of the clot.
In in vitro testing, the researchers compared various combinations of drug treatment, the use of microbubbles and ultrasound to eliminate clots, and the new technique, using nanodroplets and ultrasound.
“We found that the use of nanodroplets, ultrasound and drug treatment was the most effective, decreasing the size of the clot by 40%, plus or minus 9%,” says Xiaoning Jiang, Dean F. Duncan Distinguished Professor of Mechanical and Aerospace Engineering at NC State and corresponding author of the paper. “Using the nanodroplets and ultrasound alone reduced the mass by 30%, plus or minus 8%. The next best treatment involved drug treatment, microbubbles, and ultrasound – and that reduced clot mass by only 17%, plus or minus 9%. All these tests were conducted with the same 30-minute treatment period.
“These early test results are very promising.”
“The use of ultrasound to disrupt blood clots has been studied for years, including several substantial studies in patients in Europe, with limited success,” says co-author Paul Dayton, William R. Kenan Jr. Distinguished Professor of Biomedical Engineering at UNC and NC State. “However, the addition of the low-boiling point nanodroplets, combined with the ultrasound drill has demonstrated a substantial advance in this technology.”
“Next steps will involve pre-clinical testing in animal models that will help us assess how safe and effective this technique may be for treating deep vein thrombosis,” says Zhen Xu, a professor of biomedical engineering at the University of Michigan and co-author of the paper.
The paper, “Nanodroplet-Mediated Catheter-Directed Sonothrombolysis of Retracted Blood Clots,” is published open access in the journal Microsystems & Nanoengineering. The paper was co-authored by Huaiyu Wu and Bohua Zhang, who are Ph.D. students at NC State; and Jinwook Kim, a postdoctoral researcher in the Joint Department of Biomedical Engineering at UNC and NC State.
The work was done with support from the National Institutes of Health, under grant R01HL141967.
A startup called SonoVascular, Inc., which was co-founded by Jiang, has licensed the ultrasound “drill” technology from NC State. SonoVascular and NC State are hoping to work with industry partners to advance the technology. The low-boiling point nanodroplets, co-invented by Dayton, have also been issued a U.S. patent. That technology has been licensed by spinout company Triangle Biotechnology, Inc., which was co-founded by Dayton. Study co-authors Dayton, Kim, Xu and Jiang have also filed a patent application related to nanodroplet-mediated sonothrombolysis.
-shipman-
Note to Editors: The study abstract follows.
“Nanodroplet-Mediated Catheter-Directed Sonothrombolysis of Retracted Blood Clots”
Authors: Leela Goel, Jinwook Kim and Paul A. Dayton, North Carolina State University and University of North Carolina at Chapel Hill; Huaiyu Wu, Bohua Zhang and Xiaoning Jiang, North Carolina State University; and Zhen Xu, University of Michigan
Published: Jan. 6, Microsystems & Nanoengineering
DOI: 10.1038/s41378-020-00228-9
Abstract: One major challenge in current microbubble (MB) and tissue plasminogen activator (tPA)-mediated sonothrombolysis techniques is effectively treating retracted blood clots, owing to the high density and low porosity of retracted clots. Nanodroplets (NDs) have the potential to enhance retracted clot lysis owing to their small size and ability to penetrate into retracted clots to enhance drug delivery. For the first time, we demonstrate that a sub-megahertz, forward-viewing intravascular (FVI) transducer can be used for ND-mediated sonothrombolysis, in vitro. In this study, we determined the minimum peak negative pressure to induce cavitation with low-boiling point phase change nanodroplets and clot lysis. We then compared nanodroplet mediated sonothrombolysis to MB and tPA mediate techniques. The clot lysis as a percent mass decrease in retracted clots was 9 ± 8%, 9 ± 5%, 16 ± 5%, 14 ± 9%, 17 ± 9%, 30 ± 8%, and 40 ± 9% for the control group, tPA alone, tPA + US, MB + US, MB + tPA + US, ND + US, and ND + tPA + US groups, respectively. In retracted blood clots, combined ND- and tPA-mediated sonothrombolysis was able to significantly enhance retracted clot lysis compared with traditional MB and tPA-mediated sonothrombolysis techniques. Combined nanodroplet with tPA-mediated sonothrombolysis may provide a feasible strategy for safely treating retracted clots.