For Immediate Release
Chemical engineering researchers have developed a new catalyst that significantly increases yield in styrene manufacturing, while simultaneously reducing energy use and greenhouse gas emissions.
“Styrene is a synthetic chemical that is used to make a variety of plastics, resins and other materials,” says Fanxing Li, corresponding author of the work and Alcoa Professor of Chemical Engineering at North Carolina State University. “Because it is in such widespread use, we are pleased that we could develop a technology that is cost effective and will reduce the environmental impact of styrene manufacturing.” Industry estimates predict that manufacturers will be producing more than 33 million tons of styrene each year by 2023.
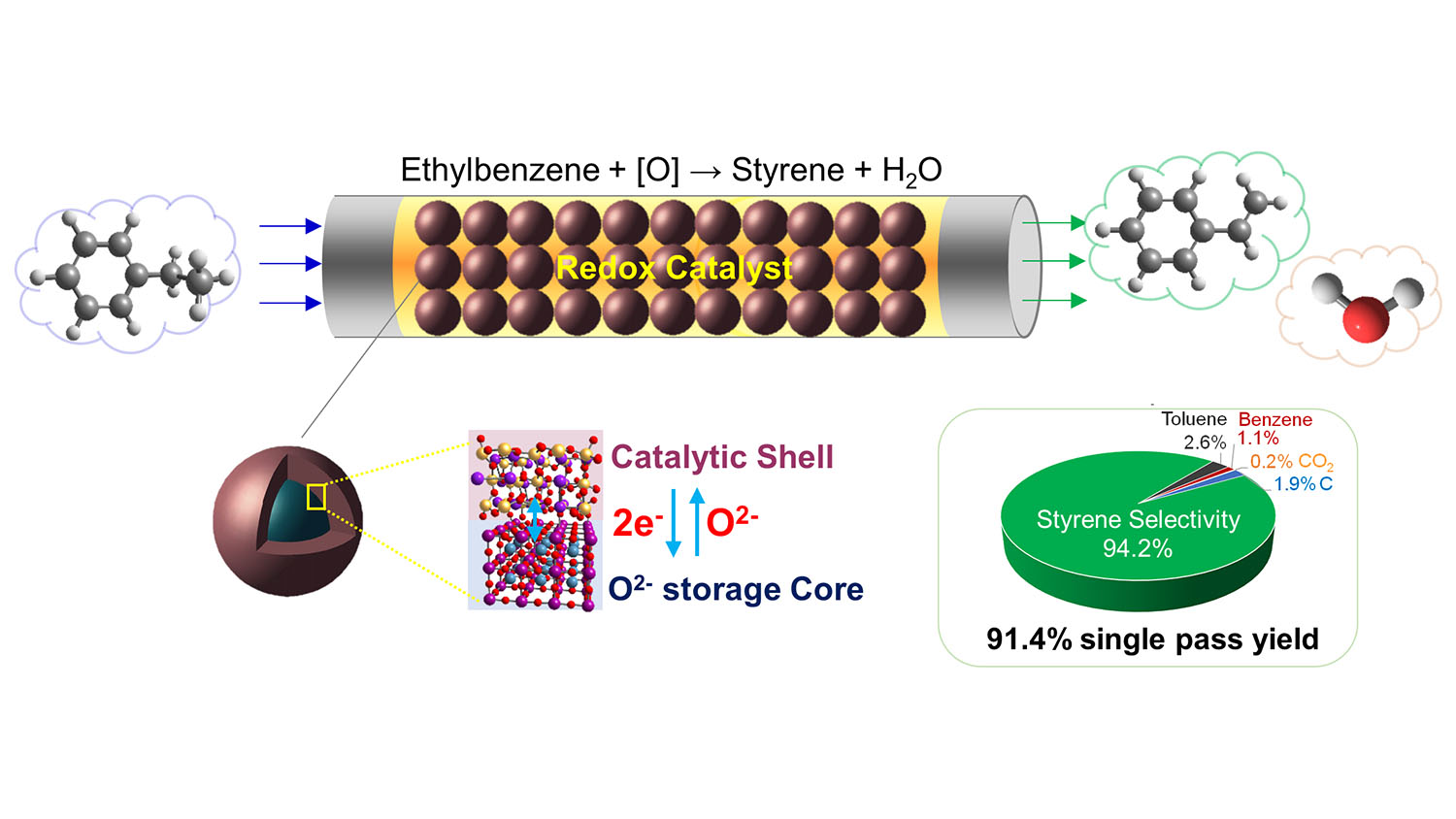
Conventional styrene production technologies have a single-pass yield of about 54%. In other words, for every 100 units of feedstock you put into the process, you would get 54 units of styrene out of each pass. Using their new catalyst, the researchers were able to achieve a single-pass yield of 91%.
The conversion process takes place at 500-600 degrees Celsius – the same temperature range as conventional styrene manufacturing processes. However, there is a big difference.
“Current techniques require injecting very large volumes of steam into the reactor where the conversion takes place,” says Yunfei Gao, a postdoctoral scholar at NC State and co-lead author of a paper on the work. “Our technique requires no steam. In practical terms, this drastically reduces the amount of energy needed to perform the conversion.”
Specifically, the conversion process that incorporates the new catalyst uses 82% less energy – and reduces carbon dioxide emissions by 79%.
“These advances are made possible by the engineered design of the catalyst itself,” says Xing Zhu, co-lead author of the paper and a researcher at the Kunming University of Science and Technology (KUST). “The new redox catalyst has a potassium ferrite surface for the catalytic phase and a mixed calcium manganese oxide core for lattice oxygen storage.” Zhu worked on the project as a visiting scholar at NC State.
“In order to adopt the new catalyst, styrene manufacturers would need to adopt a different style of reactor than they are currently using,” Li says. “They would need something similar to a CATOFIN® reactor. But those are already in widespread use for other industrial applications. And the cost savings from the new process should be significant.”
The paper, “A tailored multi-functional catalyst for ultra-efficient styrene production under a cyclic redox scheme,” is published in the journal Nature Communications. The paper is co-authored by Xijun Wang, Vasudev Haribal, Junchen Liu and Luke Neal of NC State; Zhenghong Bao and Zili Wu of Oak Ridge National Laboratory; and Hua Wang of KUST.
The research was done with support from the U.S. Department of Energy, under RAPID subaward DE-EE0007888-05-6; the National Science Foundation, under grant number 1923468; and the Kenan Institute for Engineering, Technology and Science at NC State.
The researchers have received a patent for the new technology.
-shipman-
Note to Editors: The study abstract follows.
“A tailored multi-functional catalyst for ultra-efficient styrene production under a cyclic redox scheme”
Authors: Xing Zhu, North Carolina State University and Kunming University of Science and Technology; Yunfei Gao, Xijun Wang, Vasudev Haribal, Junchen Liu, Luke M. Neal, and Fanxing Li, North Carolina State University; Zhenghong Bao and Zili Wu, Oak Ridge National Laboratory (ORNL); and Hua Wang, Kunming University of Science and Technology
Published: Feb. 26, Nature Communications
DOI: 10.1038/s41467-021-21374-2
Abstract: Styrene is an important commodity chemical that is highly energy and CO2 intensive to produce. We report a redox oxidative dehydrogenation (redox-ODH) strategy to efficiently produce styrene. Facilitated by a multifunctional (Ca/Mn)1-xO@KFeO2 core-shell redox catalyst which acts as (i) a heterogeneous catalyst, (ii) an oxygen separation agent, and (iii) a selective hydrogen combustion material, redox-ODH auto-thermally converts ethylbenzene to styrene with up to 97% single-pass conversion and >94% selectivity. This represents a 72% yield increase compared to commercial dehydrogenation on a relative basis, leading to 82% energy savings and 79% CO2 emission reduction. The redox catalyst is composed of a catalytically active KFeO2 shell and a (Ca/Mn)1-xO core for reversible lattice oxygen storage and donation. The lattice oxygen donation from (Ca/Mn)1-xO sacrificially stabilizes Fe3+ in the shell to maintain high catalytic activity and coke resistance. From a practical standpoint, the redox catalyst exhibits excellent long-term performance under industrially compatible conditions.