For Immediate Release
Researchers at North Carolina State University have developed a faster, less expensive technique for producing hindered amines – a class of chemicals used as building blocks in products ranging from pharmaceuticals and agrochemicals to detergents and organic light emitting diodes.
“Hindered amines are used in a tremendous variety of products, but all of the existing techniques for producing these amines are complicated and expensive,” says Milad Abolhasani, corresponding author of a paper on the new technique and an associate professor of chemical and biomolecular engineering at NC State. “We set out to develop a better method for synthesizing these hindered amines, and we were successful.”
One of the less expensive techniques for producing hindered amines is hydroaminomethylation, or HAM. However, the chemical industry has largely avoided using HAM, because there are too many ways things can go wrong – leaving producers with undesirable chemicals instead of the functionalized amines they were trying to make. Researchers have improved the HAM process over the years. But all of the techniques for avoiding undesirable byproducts have meant extending the timeframe of the HAM process, so that it takes hours to perform all of the necessary reactions. Until now.
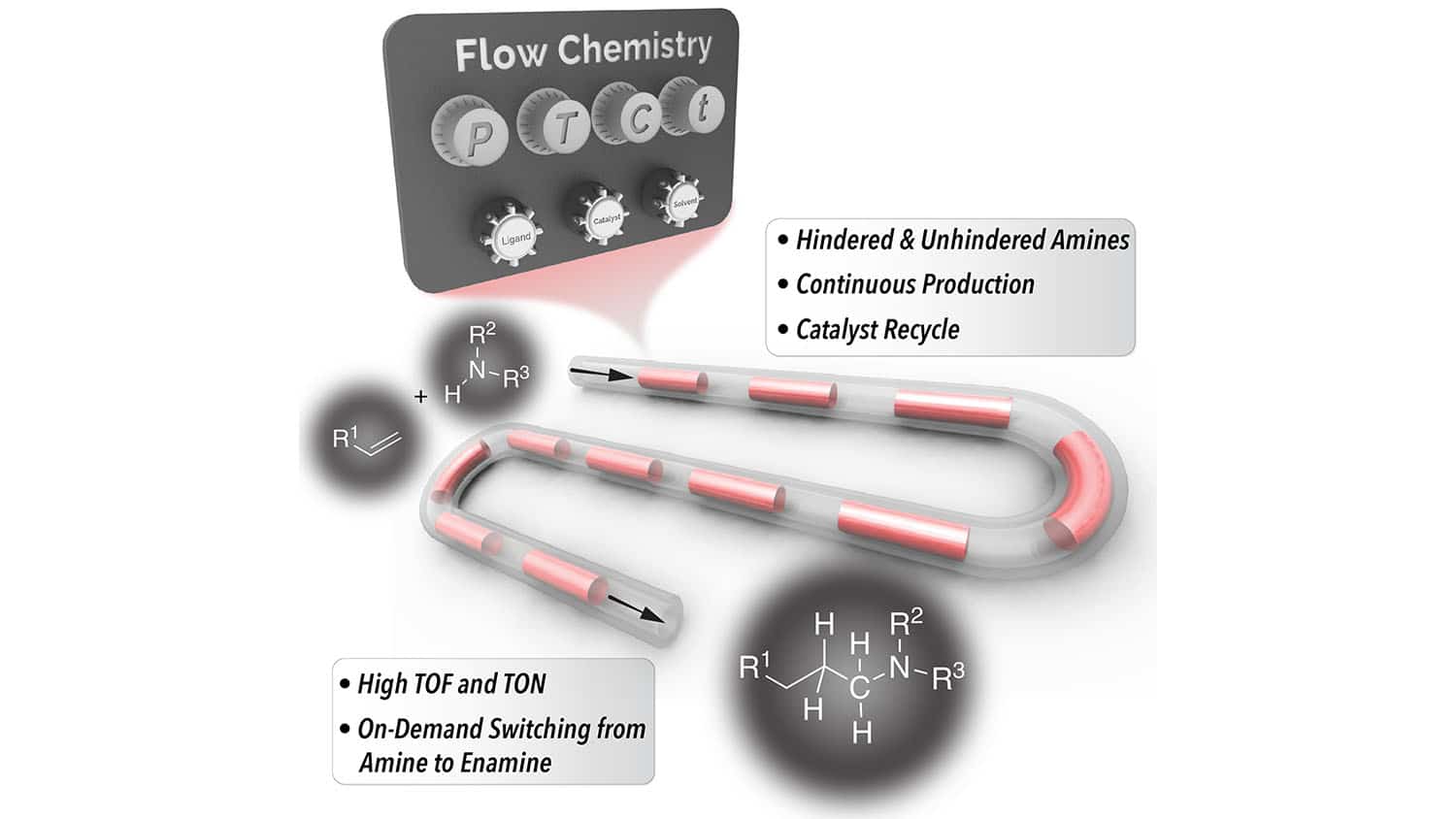
“We’ve developed a HAM technique that makes use of continuous flow reactor technologies to produce hindered amines more efficiently,” Abolhasani says. “Our HAM process takes less than 30 minutes in most cases. The only products are hindered amines and water. And we are able to recycle the primary catalyst, rhodium/N-Xantphos, which further drives down costs.”
The success of the new technique is made possible by two things. First, by using a continuous flow reactor that allows for continuous flow of both gases and liquids in a segmented flow format, the researchers were able to make the kinetics of the reaction far more efficient. Second, the new technique makes use of a co-catalyst – fluorinated benzoic acid – which reduces the amount of energy needed to perform some of the necessary reactions in the HAM process.
Ultimately, this technique drives down the cost of producing hindered amines using inexpensive feedstock, allowing users to produce them more quickly and with no toxic byproducts.
“By designing a cooperative catalyst system, we’ve demonstrated that the rate of the HAM reactions in our system can be 70 times higher than the existing state-of-the-art processes,” says Malek Ibrahim, first author of the paper and a former postdoctoral researcher at NC State. “This process is also a good example for how flow chemistry platforms can improve catalyst turnover frequency, which is increasingly important as the price of rhodium catalysts goes up.”
The new technique is particularly attractive for decentralized manufacturing operations, since the small footprint of the necessary equipment and its scalability allows users to efficiently produce hindered amines on site and on demand.
“What’s more, the same technique can also be used to produce enamines – which are other chemical building blocks – on demand, simply by tuning the solvents we use in the flow reactor,” Ibrahim says. “You can literally switch back and forth between producing amines and enamines without having to stop the production process, since the only thing you’re changing is the solvent mixture.”
The researchers have filed a provisional patent on the new technique and are now looking for industrial partners to put the technique into widespread use.
The paper, “Recyclable Cooperative Catalyst for Accelerated Hydroaminomethylation of Hindered Amines in a Continuous Segmented Flow Reactor,” is published in the journal Nature Communications. The work was done with start-up funding from NC State.
-shipman-
Note to Editors: The study abstract follows.
“Recyclable Cooperative Catalyst for Accelerated Hydroaminomethylation of Hindered Amines in a Continuous Segmented Flow Reactor”
Authors: Malek Y. S. Ibrahim and Milad Abolhasani, North Carolina State University
Published: May 4, Nature Communications
DOI: 10.1038/s41467-022-30175-0
Abstract: Synthesis of hindered amines using the atom-efficient hydroaminomethylation (HAM) route remains a challenge. Here, we report a general and accelerated HAM in segmented flow, achieved via a cooperative effect between rhodium (Rh)/N-Xantphos and a co-catalyst (2-Fluoro-4-methylbenzoic acid) to increase the reactivity by 70 fold when compared to Rh/Xantphos in batch reactors. The cooperation between Rh and the co-catalyst facilitates the cleavage of the H-H bond and drives the equilibrium-limited condensation step forward. Online reaction optimization expands the scope to include alkyl, aryl, and primary amines. In-flow solvent tuning enables selectivity switching from amine to enamine without the need for changing the ligand. Furthermore, leveraging the ionic nature of the catalyst, we present a robust Rh recovery strategy up to 4 recycles without loss of activity.