Researchers Integrate Diamond/Boron Nitride Crystalline Layers for Use in High-Power Devices
For Immediate Release
Materials researchers at North Carolina State University have developed a new technique to deposit diamond on the surface of cubic boron nitride (c-BN), integrating the two materials into a single crystalline structure.
“This could be used to create high-power devices, such as the solid state transformers needed to create the next generation ‘smart’ power grid,” says Jay Narayan, the John C. Fan Distinguished Chair Professor of Materials Science and Engineering at NC State and lead author of a paper describing the research.
“It could also be used to create cutting tools, high-speed machining and deep sea drilling equipment,” Narayan says. “Diamond is hard, but it tends to oxidize, transforming into graphite – which is softer. A coating of c-BN would prevent oxidation. Diamond also interacts with iron, making it difficult to use with steel tools. Again, c-BN would address the problem.”
C-BN is a form of boron nitride that has a cubic crystalline structure. It has similar properties to diamond, but holds several advantages: c-BN has a higher bandgap, which is attractive for use in high-power devices; c-BN can be “doped” to give it positively- and negatively-charged layers, which means it could be used to make transistors; and it forms a stable oxide layer on its surface when exposed to oxygen, making it stable at high temperatures. Earlier this year, Narayan unveiled a faster, less expensive technique for creating c-BN.
To create the epitaxial, or single crystal, diamond/c-BN structures, the researchers begin by creating a substrate of c-BN. This is done using the new technique Narayan published earlier this year. They then use a process called pulse-laser deposition – which is done at 500 degrees Celsius and an optimized atmospheric pressure – to deposit diamond on the surface of the c-BN. The pulse-laser technique allows them to control the thickness of the diamond layer.
“This is all done in a single chamber, making the process more energy- and time-efficient,” Narayan says. “You use only solid state carbon and BN, and it’s more environmentally benign than conventional techniques.”
The researchers were also able to deposit diamond on the c-BN using the conventional chemical vapor deposition technique, which utilizes methane gas, hydrogen gas and a tungsten filament at 900 °C.
“The chemical vapor deposition approach works, but our pulsed laser deposition approach works much better, doesn’t involve toxic gases, and can be done at much lower temperatures,” Narayan says.
Narayan has co-founded a company, Q-Carbon LLC, which has licensed the technique and is working to commercialize it for multiple applications.
The paper, “Direct Conversion of h-BN into c-BN and Formation of Epitaxial c-BN/Diamond Heterostructures,” is published in the Journal of Applied Physics. The paper was co-authored by Anagh Bhaumik, a Ph.D. student at NC State, and Weizong Xu, a postdoctoral research associate at NC State. The work was supported by the National Science Foundation under grant number 1304067.
-shipman-
Note to Editors: The study abstract follows.
“Direct Conversion of h-BN into c-BN and Formation of Epitaxial c-BN/Diamond Heterostructures”
Authors: Jagdish Narayan, Anagh Bhaumik, and Weizong Xu, North Carolina State University
Published: May 9, Journal of Applied Physics
DOI: 10.1063/1.4948688
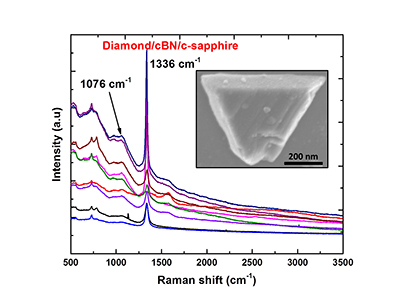
Abstract: We have created a new state of BN (named Q-BN) through rapid melting and super undercooling and quenching by using nanosecond laser pulses. Phase pure c-BN is formed either by direct quenching of super undercooled liquid or by nucleation and growth from Q-BN. Thus a direct conversion of hexagonal boron nitride (h-BN) into phase-pure cubic boron nitride (c-BN) is achieved by nanosecond pulsed laser melting at ambient temperatures and atmospheric pressure in air. According to the P-T phase diagram, the transformation from h-BN into c-BN under equilibrium processing can occur only at high temperatures and pressures, as the hBN-cBN-Liquid triple point is at 3500K/9.5GPa or 3700K/7.0GPa with a recent theoretical refinement. Using nonequilibrium nanosecond laser melting, we have created super undercooled state and shifted this triple point to as low as 2800K and atmospheric pressure. The rapid quenching from super undercooled state leads to formation of a new phase, named as Q-BN. We present detailed characterization of Q-BN and c-BN layers by using Raman spectroscopy, high-resolution scanning electron microscopy, electron-back-scatter diffraction, HRTEM and electron energy loss spectroscopy, and discuss the mechanism of formation of nanodots, nanoneedles, microneedles, and single-crystal c-BN on sapphire substrate. We have also deposited diamond by pulsed laser deposition of carbon on c-BN and created c-BN/diamond heterostructures, where c-BN acts as a template for epitaxial diamond growth. We discuss the mechanism of epitaxial c-BN and diamond growth on lattice matching c-BN template under pulsed laser evaporation of amorphous carbon, and impact of this discovery on a variety of applications.
- Categories: