NC State Researchers Advance Genome Editing Technique
For Immediate Release
Customized genome editing – the ability to edit desired DNA sequences to add, delete, activate or suppress specific genes – has major potential for application in medicine, biotechnology, food and agriculture.
Now, in a paper published in Molecular Cell, North Carolina State University researchers and colleagues examine six key molecular elements that help drive this genome editing system, which is known as CRISPR-Cas.
NC State’s Dr. Rodolphe Barrangou, an associate professor of food, bioprocessing and nutrition sciences, and Dr. Chase Beisel, an assistant professor of chemical and biomolecular engineering, use CRISPR-Cas to take aim at certain DNA sequences in bacteria and in human cells. CRISPR stands for “clustered regularly interspaced short palindromic repeats,” and Cas is a family of genes and corresponding proteins associated with the CRISPR system that specifically target and cut DNA in a sequence-dependent manner.
Essentially, the authors say, bacteria use the system as a defense mechanism and immune system against unwanted invaders such as viruses. Now that same system is being harnessed by researchers to quickly and more precisely target certain genes for editing.
“This paper sheds light on how CRISPR-Cas works,” Barrangou said. “If we liken this system to a puzzle, this paper shows what some of the system’s pieces are and how they interlock with one another. More importantly, we find which pieces are important structurally or functionally – and which ones are not.”
The CRISPR-Cas system is spreading like wildfire among researchers across the globe who are searching for new ways to manipulate genes. Barrangou says that the paper’s findings will allow researchers to increase the specificity and efficiency in targeting DNA, setting the stage for more precise genetic modifications.
The work by Barrangou and Beisel holds promise in manipulating relevant bacteria for use in food – think of safer and more effective probiotics for your yogurt, for example – and in model organisms used in agriculture, including gene editing in crops to make them less susceptible to disease.
The collaborative effort with Caribou Biosciences, a start-up biotechnology company in California, illustrates the focus of these two NC State laboratories on bridging the gap between industry and academia, and the commercial potential of CRISPR technologies, the researchers say.
– kulikowski –
Note: An abstract of the paper follows.
“Guide RNA Functional Modules Direct Cas9 Activity and Orthogonality”
Authors: Alexandra E. Briner, Kurt Selle, Chase L. Beisel and Rodolphe Barrangou, North Carolina State University; Paul D. Donohoue, Euan M. Slorach, Christopher H. Nye, Rachel E. Haurwitz, and Andrew P. May, Caribou Biosciences Inc.; Ahmed A. Gomaa, University of Cairo
Published: Oct. 16, 2014, online in Molecular Cell
DOI: 10.1016/j.molcel.2014.09.019
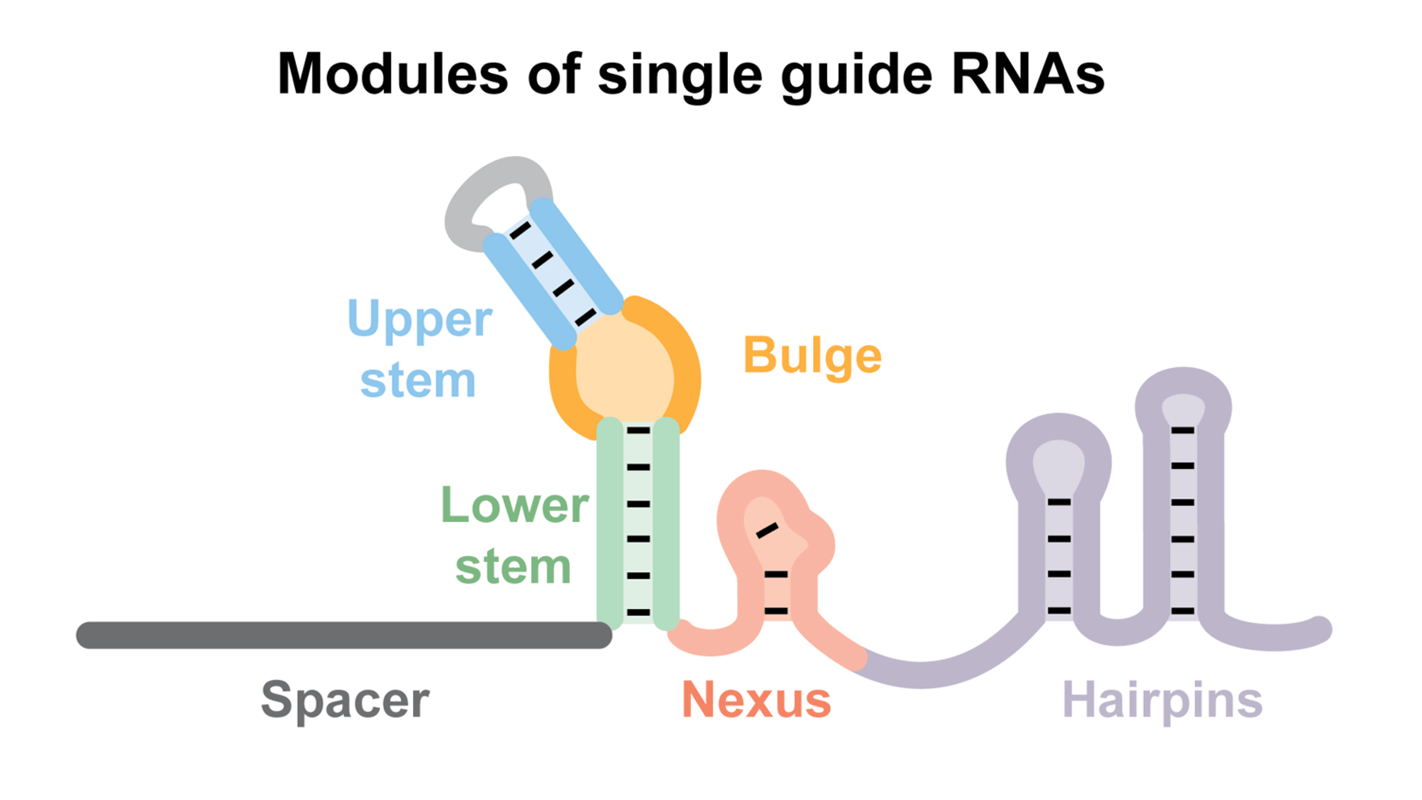
Abstract: The RNA-guided Cas9 endonuclease specifically targets and cleaves DNA in a sequence-dependent manner and has been widely used for programmable genome editing. Cas9 activity is dependent on interactions with guide RNAs, and evolutionarily divergent Cas9 nucleases have been shown to work orthogonally. However, the molecular basis of selective Cas9:guide-RNA interactions is poorly understood. Here, we identify and characterize six conserved modules within native crRNA:tracrRNA duplexes and single guide RNAs (sgRNAs) that direct Cas9 endonuclease activity. We show the bulge and nexus are necessary for DNA cleavage and demonstrate that the nexus and hairpins are instrumental in defining orthogonality between systems. In contrast, the crRNA:tracrRNA complementary region can be modified or partially removed. Collectively, our results establish guide RNA features that drive DNA targeting by Cas9 and open new design and engineering avenues for CRISPR technologies.
- Categories:


