Microscopy Advance Reveals Unexpected Role for Water in Energy Storage Material
For Immediate Release
A material with atomically thin layers of water holds promise for energy storage technologies, and researchers have now discovered that the water is performing a different role than anyone anticipated. The finding was possible due to a new atomic force microscopy (AFM) method that measures the sub-nanoscale deformation rate in the material in response to changes in the material caused by energy storage.
The researchers studied crystalline tungsten oxide dihydrate, which consists of crystalline tungsten oxide layers separated by atomically thin layers of water. The material is of interest because it holds promise for helping to store and release energy quickly and efficiently. However, it has not been clear what role the water plays in this process.
To address this question, researchers from North Carolina State University, the Oak Ridge National Laboratory (ORNL) and Texas A&M University used a new methodology. The new technique relies on AFM to track the expansion and contraction of the material at the atomic scale and in real time as an electronic instrument called a potentiostat moves charge in and out of the material. This technique allowed the team to detect even minor deformations in the material as charge moved through it.
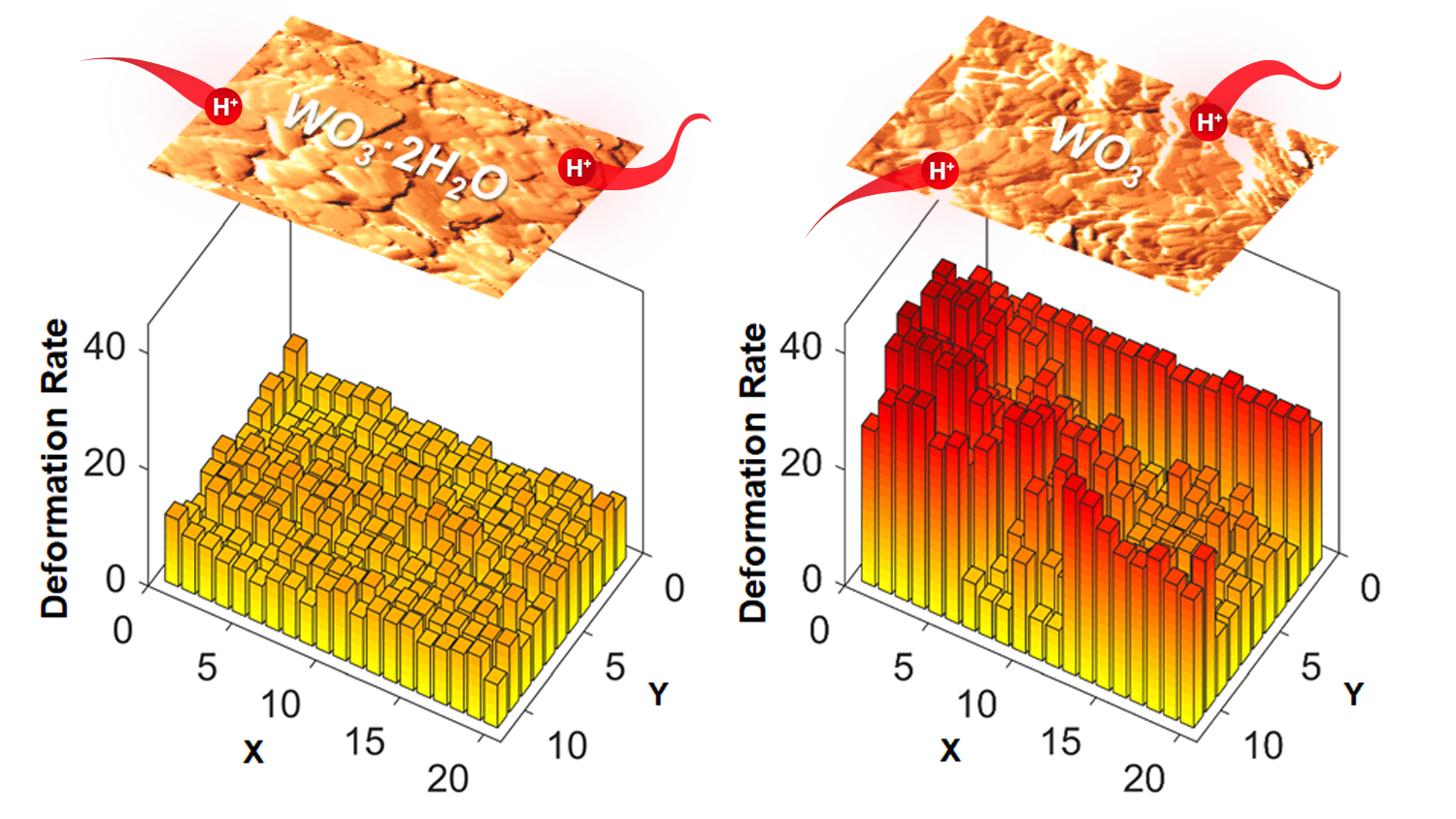
“We tested both crystalline tungsten oxide dihydrate and crystalline tungsten oxide – which lacks the water layers,” says Veronica Augustyn, an assistant professor of materials science and engineering at NC State and corresponding author of a paper on the work. “And we found that the water layers appear to play a significant role in how the material responds mechanically to energy storage.”
“Specifically, we found that the water layers do two things,” says Ruocun “John” Wang, a Ph.D. student in Augustyn’s lab and lead author of the paper. “One, the water layers minimize deformation, meaning that the material expands and contracts less as ions move in and out of the material when there are water layers. Two, the water layers make the deformation more reversible, meaning that the material returns to its original dimensions more easily.”
“In practical terms, this means that the material with water layers is more efficient at storing charge, losing less energy,” Augustyn says.
The paper, “Operando Atomic Force Microscopy Reveals Mechanics of Structural Water Driven Battery-to-Pseudocapacitor Transition,” is published in the journal ACS Nano. The paper was co-authored by James Mitchell and Shelby Boyd of NC State; Qiang Gao, Wan-Yu Tsai and Nina Balke of ORNL; and Matt Pharr of Texas A&M. The work was done with support from the National Science Foundation under grants 1653827 and 571800; an ORAU Ralph E. Powe Junior Faculty Enhancement Award, as well as support from the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility and the Fluid Interface Reactions, Structures and Transport (FIRST) Center, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences.
-shipman-
Note to Editors: The study abstract follows.
“Operando Atomic Force Microscopy Reveals Mechanics of Structural Water Driven Battery-to-Pseudocapacitor Transition”
Authors: Ruocun Wang, James B. Mitchell, Shelby Boyd and Veronica Augustyn, North Carolina State University; Qiang Gao, Wan-Yu Tsai and Nina Balke, Oak Ridge National Laboratory; and Matt Pharr, Texas A&M University
Published: May 16, ACS Nano
DOI: 10.1021/acsnano.8b02273
Abstract: The presence of structural water in tungsten oxides leads to a transition in the energy storage mechanism from battery-type intercalation (limited by solid state diffusion) to pseudocapacitance (limited by surface kinetics). Here, we demonstrate that these electrochemical mechanisms are linked to the mechanical response of the materials during intercalation of protons and present a pathway to utilize the mechanical coupling for local studies of electrochemistry. Operando atomic force microscopy dilatometry is used to measure the deformation of redox-active energy storage materials and to link the local nanoscale deformation to the electrochemical redox process. This technique reveals that the local mechanical deformation of the hydrated tungsten oxide is smaller and more gradual than the anhydrous oxide and occurs without hysteresis during the intercalation and deintercalation processes. The ability of layered materials with confined structural water to minimize mechanical deformation likely contributes to their fast energy storage kinetics.
- Categories:


