New Microneedle Technique Speeds Plant Disease Detection

For Immediate Release
Researchers have developed a new technique that uses microneedle patches to collect DNA from plant tissues in one minute, rather than the hours needed for conventional techniques. DNA extraction is the first step in identifying plant diseases, and the new method holds promise for the development of on-site plant disease detection tools.
“When farmers detect a possible plant disease in the field, such as potato late blight, they want to know what it is right away; rapid detection can be important for addressing plant diseases that spread quickly,” says Qingshan Wei, an assistant professor of chemical and biomolecular engineering at North Carolina State University and co-corresponding author of a paper on the work.
“One of the obstacles to rapid detection is the amount of time it takes to extract DNA from a plant sample, and our technique provides a fast, simple solution to that problem,” Wei says.
“Some plant diseases have similar leaf symptoms, such as late blight caused by the famed Irish famine pathogen Phytophthora infestans, and Phytophthora blight caused by a sister species P. nicotianae,” says Jean Ristaino, William Neal Reynolds Distinguished Professor of Plant Pathology at NC State and co-corresponding author of the paper. “The gold standard for disease identification is a molecular assay. Our new technique is important because you can’t run an amplification or genotyping assay on strains of P. infestans, or any other plant disease, until you’ve extracted DNA from the sample.”
Typically, DNA is extracted from a plant sample using a method called CTAB extraction, which has to be done in a lab, requires a lot of equipment, and takes at least 3 to 4 hours. CTAB extraction is a multi-step process involving everything from tissue grinding to organic solvents and centrifuges.
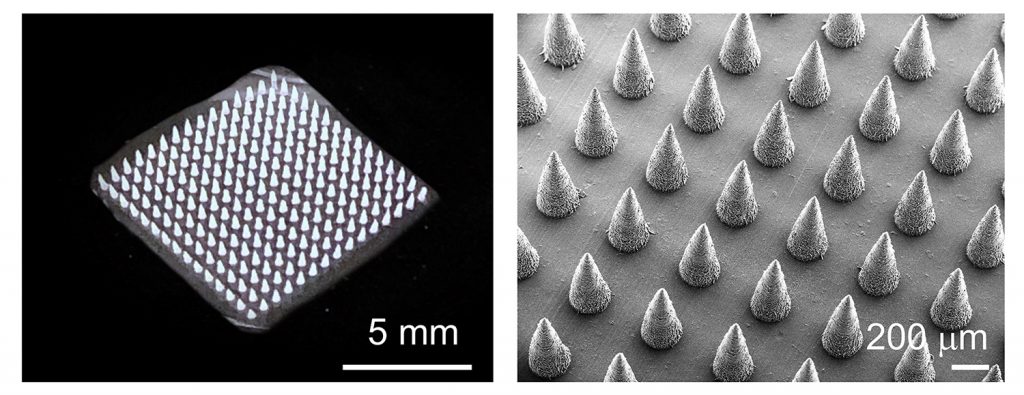
By contrast, the new DNA extraction technique involves only a microneedle patch and an aqueous buffer solution. The patch is about the size of a postage stamp and is made of an inexpensive polymer. The surface on one side of the patch is made up of hundreds of needles that are only 0.8 millimeters long.
A farmer or researcher can apply the microneedle patch to a plant they suspect is diseased, hold the patch in place for a few seconds, then peel it off. The patch is then rinsed with the buffer solution, washing genetic material off of the microneedles and into a sterile container. The entire process takes about a minute.
“It is exciting to see the new application of microneedle patch technology in agriculture and plant science,” says Zhen Gu, a professor of bioengineering at the University of California, Los Angeles and co-corresponding author of the paper, who developed several microneedle-based drug delivery systems for human health.
“In experimental testing, we found that the microneedle technique does result in slightly higher levels of impurities in the sample, as compared to CTAB,” Wei says. “However, the microneedle technique’s purity levels were comparable to other, validated laboratory methods of DNA extraction. Most importantly, we found that the slight difference in purity levels between the microneedle and CTAB samples did not interfere with the ability to accurately test the samples by a PCR or LAMP assay.”
“The fact that microneedles extract a smaller sampling volume seems not to be an issue,” says Rajesh Paul, a Ph.D. student at NC State and first author of the paper. “The microneedle technique successfully extracted pathogen DNA from all field-collected infected tomato leaves in a recent blind test.”
“DNA extraction has been a significant hurdle to the development of on-site testing tools,” Wei says. “We are now moving forward with the goal of creating an integrated, low-cost, field-portable device that can perform every step of the process from taking the sample to identifying the pathogen and reporting the results of an assay.”
The paper, “Extraction of Plant DNA by Microneedle Patch for Rapid Detection of Plant Diseases,” is published in the journal ACS Nano. The paper was co-authored by Amanda Saville, lab manager in Ristaino’s lab; Jeana Hansel, a graduate student at NC State; Carmin Ball and Alyssa Williams, undergraduates at NC State; Yanqi Ye, a former graduate student in the Joint Biomedical Engineering Department at NC State and the University of North Carolina, Chapel Hill; Xinyuan Chang, an undergraduate at Tianjin University who worked as a visiting researcher at NC State; and Guojun Chen, postdoctoral researcher at UCLA.
The work was done with support from the NC State Chancellor’s Faculty Excellence Program on Emerging Plant Disease and Global Food Security, the Kenan Institute for Engineering, Technology & Science at NC State, and the USDA Integrated Pest Information Platform for Extension and Education, under grant number 2015-0097.
-shipman-
Note to Editors: The study abstract follows.
“Extraction of Plant DNA by Microneedle Patch for Rapid Detection of Plant Diseases”
Authors: Rajesh Paul, Amanda C. Saville, Jeana C. Hansel, Carmin Ball, Alyssa Williams, Jean B. Ristaino and Qingshan Wei, North Carolina State University; Yanqi Ye, Joint Biomedical Engineering Department at North Carolina State University and the University of North Carolina, Chapel Hill; Xinyuan Chang, Tianjin University; Guojun Chen and Zhen Gu, University of California, Los Angeles
Published: June 9, ACS Nano
DOI: 10.1021/acsnano.9b00193
Abstract: In-field molecular diagnosis of plant diseases via nucleic acid amplification (NAA) is currently limited by cumbersome protocols for extracting and isolating pathogenic DNA from plant tissues. To address this challenge, a rapid plant DNA extraction method has been developed using a disposable polymeric microneedle (MN) patch. By applying MN patches on plant leaves, amplification-assay-ready DNA can be extracted within a minute from different plant species. MN-extracted DNA has been used for direct polymerase chain reaction (PCR) amplification of plant plastid DNA without purification. Furthermore, using this patch device, extraction of plant pathogen DNA (Phytophthora infestans) from both laboratory-inoculated and field-infected leaf samples was performed for detection of late blight disease in tomato. MN extraction achieved 100% detection rate of late blight infections for samples after 3 days of inoculation when compared to the conventional cetyltrimethylammonium bromide (CTAB)-based DNA extraction method as a gold standard, and 100% detection rate for all blind field samples tested. This simple, cell lysis-free, and purification-free DNA extraction method could be a transformative approach to facilitate rapid sample preparation for molecular diagnosis of various plant diseases directly in the field.


